Tweet
This week and next we are going to discuss another arthropod-borne infection that occurs differently in different hemispheres of the world, i.e Africa and the Americas. In Africa, the disease, while heterogenous from west to east Africa, is often fatal within a year of becoming infected and is known as sleeping sickness. In the Americas, the disease is more typically associated with long-term chronic infection that ultimately leads to premature death due to cardiac failure.
Like malaria, trypanosomiasis is caused by protozoan parasites. But rather than Plasmodium species, the pathogenic parasites in this case are trypanosomes. Trypanosomes are in the family Mastigophora, which means that they are flagellated protozoa. Flagellated protozoa have a flagellum, which is a long tail-like structure that provides motility to the parasite. In addition to the flagellum, trypanosomes have undulating membranes that extend laterally down the length of the organism. This structure provides further motility to the parasite. Here are some pictures that highlight the unique structures of trypanosomes:
Notice the flagellum and undulating membrane structures. This particular flagellated protozoan is further classified as a hemoflagellate, because it is transmitted by blood-sucking insects. The hemoflagellates include both the trypanosoma as well as the leishmania (which I will cover following the posts on trypanosomes).
So, trypanosomiasis, both the African and American forms, are caused by the hemoflagellate protozoan parasite of the genus Tyrpanosoma. Below is a picture depicting different morphological forms that kinetoplastids (a group of flagellated protozoa that includes both Trypanosoma and Leishmania) can express through different stages of development. This will be useful as a reference as we discuss the life cycles of the different Trypanosoma species :
The parent species that causes the African disease is Trypanosoma brucei, and the parent species that causes the American disease is T. cruzi. We will consider T. brucei and it's disease, African trypanosomiasis in this post, and T. cruzi and American trypanosomiasis in next week's post.
African trypanosomiasis, or sleeping sickness as it is more commonly known, can be a debilitating and deadly disease if left untreated. As mentioned above it is caused by T. brucei, which requires a vector for transmission to humans. The vector for T. brucei is the tsetse fly (Glossina spp.).
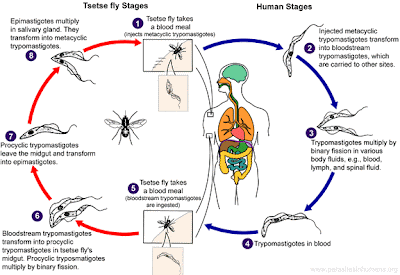
Relative to the other protozoan parasites (Plasmodium) we've discussed so far at Infection Landscapes, T. brucei has a more straightforward life cycle. The trypanosomes are introduced into the human host by injection when the tsetse fly takes its blood meal. Metacyclic trypomastigotes are injected into the host during the fly's feeding. These then transform into bloodstream trypomastigotes, which can then be transported to more distal sites within the host. These trypomastigotes proliferate by binary fission in blood, lymph and cerebral spinal fluid (CSF). Those that remain in blood circulation can be taken up during subsequent blood meals from different tsetse flies. When the bloodstream trypomastigotes find themselves in the new environment of the fly gut, they transform into procyclic trypomastigotes. At this point, the parasite again proliferates through binary fission. The procyclic trypomastigotes then migrate out of the fly gut and transform into epimastigotes, which will multiply in the salivary glands of the fly. Finally, as they proliferate in the salivary glands, the epimastigotes will transform one last time into the metacyclic trypomastigotes, which are the infectious stage for humans and can initiate new infections as the fly takes its next blood meal. The Centers for Disease Control and Prevention (CDC) have produced a nice graph depicting the different stages of the life cycle for T. brucei:
This is the general life cycle of T. brucei. While the life cycle is fairly constant across subspecies of T. brucei, the subspecies do vary dramatically across geography in sub-Saharan Africa, and these spatial distinctions correspond to differences in the manifestation and severity of disease. T. brucei gambiense occurs in West Africa and is associated with slower progression of disease. T. brucei rhodesiense occurs in East Africa and is associated with more rapid progression of disease. Below is a nice map produced by Dickson Despommier at medicalecology.org, which depicts this geographic boundary as well as the level of endemicity of infection:
These geographic distinctions in parasite distribution and disease occurrence are largely demarcated by the landscapes that define different ecologies for Glossina species. The picture below depicts the most important Glossina species for human trypanosome transmission. The fly series on the left shows the different developmental stages of Glossina palpalis, which is the riverine tsetse fly, with the adult fly at the top. The fly series on the right shows the different developmental stages of Glossina morsitans, which is the savannah tsetse fly, again with the adult fly at the top.
G. palpalis, the river tsetse fly, is, not surprisingly, associated with riverine environments. This fly has an extremely high population density along its river corridors, and is thus responsible for high levels of exposure to trypanosomes. Interestingly, though, this fly is limited in its distribution to this specific environment. If you travel only a couple hundred yards back from the river the fly's population density drops dramatically. In fact, G. palpalis is so closely tied to its narrow river habitat that it only serves as an efficient vector for trypanosomiasis within this very narrow landscape. As such, moving human settlements back from rivers can be an important control measure in reducing T. brucei gambiense transmission. However, because of the fact that many human activities (e.g., obtaining water, bathing, washing clothes, fishing, recreation) occur in the water or at the water's edge, eliminating transmission, particularly when fly density is so high, remains very difficult.
G. morsitans, the savannah tsetse fly, is associated with savannah environments. Occupying principally savannah, savannah scrub, and savannah forest habitat, this fly is much more dispersed than G. palpalis, which has a much narrower ecologic niche. However, even though G. morsitans is more robust to the landscape grades in its habitat, it is also very sparse. Indeed, field surveys have shown that the population density of this tsetse fly is approximately 1 fly per square kilometer. So, while its distribution is spatially quite broad, its very low density in any one place determine that this fly's efficiency as a vector is low.
Here are two short videos describing the life cycle of the tsetse fly.
Part 1:
Part 2;
Let's now discuss the disease itself. During the first phase of the disease the trypanosome parasites migrate through the blood to the lymph nodes, which triggers ongoing attacks of fever. These attacks can also be accompanied by headache and sometimes arthralgia. The attacks are often intermittent and can recur over a period of weeks to months. Swelling of the lymph nodes is common, and the appearance of large swelling of the lymph nodes at the back of the neck (known as Winterbottom's sign) is a strong indicator of infection:
Sustained infection can lead to cardiopathy, nephropathy, and anemia, but these conditions do not typically lead to death before the second phase of the disease ensues. Typically in African trypanosomiasis the parasites invade the central nervous system (CNS) as the disease progresses to this second, neurologic, phase. Involvement of the CNS can cause meningoencephalitis as well as associated mental confusion and disrupted sleeping habits. Prolonged diurnal sleeping is the condition from which this trypanosomiasis derives its common name. Without treatment, this neurologic condition will eventually lead to coma and death.
There are widely varying estimates of the disease burden of African trypanosomiasis because surveillance is very poor in remote areas. Nevertheless, some surveys have been undertaken with incidence estimates ranging between 10,000 and 500,000 new cases per year across the whole of the African continent. The map below by Dickson Despommier again highlights those countries with the greatest burden of disease:
The most extensive attempt to date at mapping the distribution and burden of African trypanosomiasis was published here last year in the International Journal of Health Geographics. The continent-wide distribution mapped in this study is presented below:
This map follows the expected lines of demarcation for the occurrence of T. brucei gambiense and T. brucei rhodesiense, and also suggests a high occurrence of disease. It is important to keep in mind that the cases in this map only include those that were reported or captured, and so comprise a minority of the total cases.
This last map below depicts the number of deaths per 100,000 population due to African trypanosomiasis in 2002. As you can see, the mortality associated with this disease presents a substantial burden in several countries:
This concludes the first half of our discussion on trypanosomiasis. Next week I will cover the other form of this disease, American trypanosomiasis, or Chagas disease, which is caused by a different species of Trypanosoma, i.e. T. cruzi.
Stay tuned.
This week and next we are going to discuss another arthropod-borne infection that occurs differently in different hemispheres of the world, i.e Africa and the Americas. In Africa, the disease, while heterogenous from west to east Africa, is often fatal within a year of becoming infected and is known as sleeping sickness. In the Americas, the disease is more typically associated with long-term chronic infection that ultimately leads to premature death due to cardiac failure.
Like malaria, trypanosomiasis is caused by protozoan parasites. But rather than Plasmodium species, the pathogenic parasites in this case are trypanosomes. Trypanosomes are in the family Mastigophora, which means that they are flagellated protozoa. Flagellated protozoa have a flagellum, which is a long tail-like structure that provides motility to the parasite. In addition to the flagellum, trypanosomes have undulating membranes that extend laterally down the length of the organism. This structure provides further motility to the parasite. Here are some pictures that highlight the unique structures of trypanosomes:
Notice the flagellum and undulating membrane structures. This particular flagellated protozoan is further classified as a hemoflagellate, because it is transmitted by blood-sucking insects. The hemoflagellates include both the trypanosoma as well as the leishmania (which I will cover following the posts on trypanosomes).
So, trypanosomiasis, both the African and American forms, are caused by the hemoflagellate protozoan parasite of the genus Tyrpanosoma. Below is a picture depicting different morphological forms that kinetoplastids (a group of flagellated protozoa that includes both Trypanosoma and Leishmania) can express through different stages of development. This will be useful as a reference as we discuss the life cycles of the different Trypanosoma species :
The parent species that causes the African disease is Trypanosoma brucei, and the parent species that causes the American disease is T. cruzi. We will consider T. brucei and it's disease, African trypanosomiasis in this post, and T. cruzi and American trypanosomiasis in next week's post.
African trypanosomiasis, or sleeping sickness as it is more commonly known, can be a debilitating and deadly disease if left untreated. As mentioned above it is caused by T. brucei, which requires a vector for transmission to humans. The vector for T. brucei is the tsetse fly (Glossina spp.).
Relative to the other protozoan parasites (Plasmodium) we've discussed so far at Infection Landscapes, T. brucei has a more straightforward life cycle. The trypanosomes are introduced into the human host by injection when the tsetse fly takes its blood meal. Metacyclic trypomastigotes are injected into the host during the fly's feeding. These then transform into bloodstream trypomastigotes, which can then be transported to more distal sites within the host. These trypomastigotes proliferate by binary fission in blood, lymph and cerebral spinal fluid (CSF). Those that remain in blood circulation can be taken up during subsequent blood meals from different tsetse flies. When the bloodstream trypomastigotes find themselves in the new environment of the fly gut, they transform into procyclic trypomastigotes. At this point, the parasite again proliferates through binary fission. The procyclic trypomastigotes then migrate out of the fly gut and transform into epimastigotes, which will multiply in the salivary glands of the fly. Finally, as they proliferate in the salivary glands, the epimastigotes will transform one last time into the metacyclic trypomastigotes, which are the infectious stage for humans and can initiate new infections as the fly takes its next blood meal. The Centers for Disease Control and Prevention (CDC) have produced a nice graph depicting the different stages of the life cycle for T. brucei:
This is the general life cycle of T. brucei. While the life cycle is fairly constant across subspecies of T. brucei, the subspecies do vary dramatically across geography in sub-Saharan Africa, and these spatial distinctions correspond to differences in the manifestation and severity of disease. T. brucei gambiense occurs in West Africa and is associated with slower progression of disease. T. brucei rhodesiense occurs in East Africa and is associated with more rapid progression of disease. Below is a nice map produced by Dickson Despommier at medicalecology.org, which depicts this geographic boundary as well as the level of endemicity of infection:
These geographic distinctions in parasite distribution and disease occurrence are largely demarcated by the landscapes that define different ecologies for Glossina species. The picture below depicts the most important Glossina species for human trypanosome transmission. The fly series on the left shows the different developmental stages of Glossina palpalis, which is the riverine tsetse fly, with the adult fly at the top. The fly series on the right shows the different developmental stages of Glossina morsitans, which is the savannah tsetse fly, again with the adult fly at the top.
G. palpalis, the river tsetse fly, is, not surprisingly, associated with riverine environments. This fly has an extremely high population density along its river corridors, and is thus responsible for high levels of exposure to trypanosomes. Interestingly, though, this fly is limited in its distribution to this specific environment. If you travel only a couple hundred yards back from the river the fly's population density drops dramatically. In fact, G. palpalis is so closely tied to its narrow river habitat that it only serves as an efficient vector for trypanosomiasis within this very narrow landscape. As such, moving human settlements back from rivers can be an important control measure in reducing T. brucei gambiense transmission. However, because of the fact that many human activities (e.g., obtaining water, bathing, washing clothes, fishing, recreation) occur in the water or at the water's edge, eliminating transmission, particularly when fly density is so high, remains very difficult.
G. morsitans, the savannah tsetse fly, is associated with savannah environments. Occupying principally savannah, savannah scrub, and savannah forest habitat, this fly is much more dispersed than G. palpalis, which has a much narrower ecologic niche. However, even though G. morsitans is more robust to the landscape grades in its habitat, it is also very sparse. Indeed, field surveys have shown that the population density of this tsetse fly is approximately 1 fly per square kilometer. So, while its distribution is spatially quite broad, its very low density in any one place determine that this fly's efficiency as a vector is low.
Here are two short videos describing the life cycle of the tsetse fly.
Part 1:
Part 2;
Let's now discuss the disease itself. During the first phase of the disease the trypanosome parasites migrate through the blood to the lymph nodes, which triggers ongoing attacks of fever. These attacks can also be accompanied by headache and sometimes arthralgia. The attacks are often intermittent and can recur over a period of weeks to months. Swelling of the lymph nodes is common, and the appearance of large swelling of the lymph nodes at the back of the neck (known as Winterbottom's sign) is a strong indicator of infection:
Sustained infection can lead to cardiopathy, nephropathy, and anemia, but these conditions do not typically lead to death before the second phase of the disease ensues. Typically in African trypanosomiasis the parasites invade the central nervous system (CNS) as the disease progresses to this second, neurologic, phase. Involvement of the CNS can cause meningoencephalitis as well as associated mental confusion and disrupted sleeping habits. Prolonged diurnal sleeping is the condition from which this trypanosomiasis derives its common name. Without treatment, this neurologic condition will eventually lead to coma and death.
There are widely varying estimates of the disease burden of African trypanosomiasis because surveillance is very poor in remote areas. Nevertheless, some surveys have been undertaken with incidence estimates ranging between 10,000 and 500,000 new cases per year across the whole of the African continent. The map below by Dickson Despommier again highlights those countries with the greatest burden of disease:
The most extensive attempt to date at mapping the distribution and burden of African trypanosomiasis was published here last year in the International Journal of Health Geographics. The continent-wide distribution mapped in this study is presented below:
This map follows the expected lines of demarcation for the occurrence of T. brucei gambiense and T. brucei rhodesiense, and also suggests a high occurrence of disease. It is important to keep in mind that the cases in this map only include those that were reported or captured, and so comprise a minority of the total cases.
This last map below depicts the number of deaths per 100,000 population due to African trypanosomiasis in 2002. As you can see, the mortality associated with this disease presents a substantial burden in several countries:
This concludes the first half of our discussion on trypanosomiasis. Next week I will cover the other form of this disease, American trypanosomiasis, or Chagas disease, which is caused by a different species of Trypanosoma, i.e. T. cruzi.
Stay tuned.