Tweet
Few words are as emotive in the world of public health as the Plague. Indeed, it is unique among diseases in that the invocation of its very name demands not only the name itself, but also the definite article. So embedded in the depths of the human imagination, this malady requires all others to stand upon the order of its own coming and going. The Plague. Even the font seems to shift and contort into grotesque features as you read the written word upon the page. The fixed and fantastically dull, Times New Roman, is transformed before your eyes into something only the fear of the memory of countless generations can transcribe:
The Plague.
But enough of hyperbole, why should this disease evoke such profound dread? First and foremost, it does not constitute a large-scale public health burden, or threat, so why the fuss? HIV/AIDS, tuberculosis, and malaria are the big three, which account for miillions of lives lost and immeasurable morbidity each year around the world. None of these, not even HIV as we currently live through its devastating pandemic, invoke the visceral response of plague. The loathsome neglected tropical diseases such as trypanosomiasis and leishmaniasis, which constitute large burdens across the developing world, can inflict horrible morbidity and mortality that devastates communities, but even these typically are not feared like the plague. The yearly varied influenza virus in all its forms, constantly renewing itself each season, does not even motivate most to vaccinate! The emergence of the superbugs, those virulent bacteria that have developed resistance to antibiotic therapy such as methicillin-resistant staphylococcus aureus, may instill an unequaled fear among infectious disease clinicians (quite justified, I might add), but they do not, generally, strike fear into the hearts of the general populace. Indeed, "plague" has such powerful connotation, that it has come to be used to refer to any severe epidemic of infectious disease.
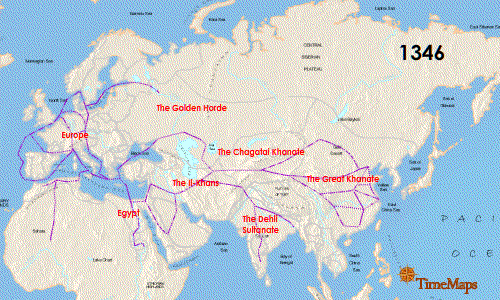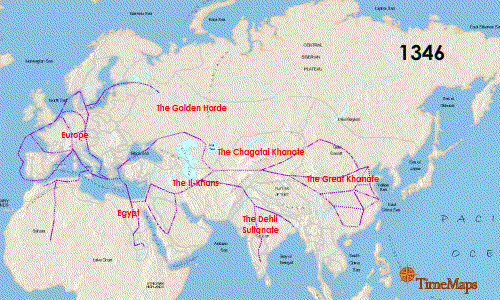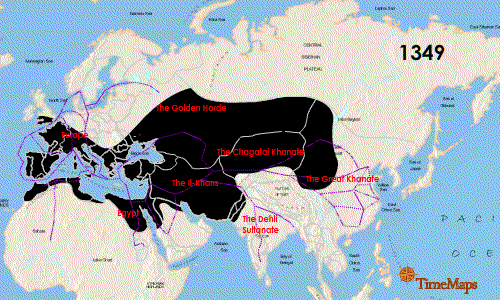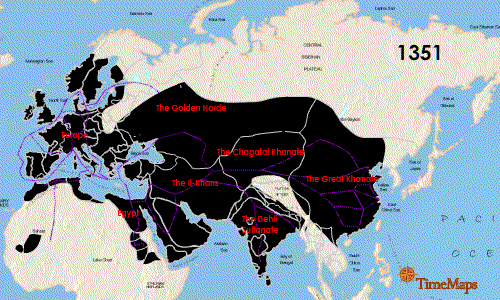
So, wherefore this dread? Likely it comes down through our shared historical experience as human beings, and what was probably one of the first instances of a human pandemic (other early pandemics were probably due to smallpox). In fact, historically, there have been three plague pandemics. The First plague pandemic occurred from 541 to 542 AD and is known as the Justinian Plague, so called because it ravaged the Byzantine Empire under the rule of the emperor Justinian. Geographically speaking, it affected the Mediterranean, including southern Europe, northern Africa, and the near-Middle East, shown here in red:
Key to the emergence of this, perhaps first, pandemic disease (though, in reality this was probably second to a smallpox pandemic during the preceding Roman empire), were the extensive trade routes across very large geographic landscapes. As people traversed large geographic spaces by land and sea, they brought their trade goods with them...and their rats...and their rats' fleas. And so the widespread dissemination of plague was facilitated by trade.
The Second plague pandemic raged from 1347 to 1351 and is known as the "Black Death". It originated in central Asia and spread rapidly following the Silk Road: once again the pandemic emerged by way of the movement of people and their rodents along the routes of trade. The spatial-temporal spread of the Second pandemic is detailed nicely in this animated graphic:
This pandemic was truly devastating. It is believed that more than 100 million people died from this plague, which represented almost 25% of the global human population. No pandemic since has claimed the lives of such a large proportion of the human population. In addition, this pandemic affected very large areas, particularly China and Europe, where the infection killed about 1/2 and 1/3 of the populations, respectively. In these regions the incidence of pneumonic plague became relatively high as the infection became more established in humans. As such, human-to-human transmission became much more prominent, which is not typically how humans acquire the infection. It is worth restating the mortality above: this pandemic killed 1/2 the population of China and 1/3 the population of Europe. It also killed 1/8 the population of Africa. The scope of such a catastrophe is utterly incomprehensible to the modern world. All we can do is stare in amazement at the staggering statistics.
Perhaps with an historical perspective, we can start to get a sense of why this disease has been so feared throughout the human experience.
We are currently living through the Third plague pandemic. This began in 1855 in China and has killed more than 12 million people there and in India over the last one and a half centuries. This pandemic has spread to each continent with permanent human populations (i.e. excluding Antarctica). It was widely distributed as bubonic plague by shipping routes, with the domestic rats carried aboard shipping vessels transporting the plague bacteria to ports all around the world. Once again, plague followed the trade routes. East, Southeast and South Asia were widely affected up until the turn of the 19th century. The plague reached the United States in 1900 and accounted for urban plague outbreaks up until the 1920s. As described in detail below, currently the world's largest remaining sylvan focus of plague continues to exist in the western and southwestern US. Though most of the human cases do not come from there. Today the largest number of human cases (over 99%) come from sub-Saharan African countries. The Democratic Republic of Congo and Madagascar both see a particularly large number of cases, relatively speaking, as it is still a rare disease. One of the most striking demonstrations of the popular fear of plague that remains into modern times comes from Surat, India. In 1994 an outbreak of pneumonic plague resulted in 54 deaths in the town of Surat in Gujarat, India. During this outbreak, a staggering 300,000 people fled the area as the news of epidemic broke. This massive popular migration brought the local economy and social infrastructure to a standstill and created a humanitarian disaster. The epidemic also affected the larger national Indian economy because of the response of the international community and the subsequent effect on India's industries. Nevertheless, the Indian public health authorities should be applauded for controlling the situation quickly and efficiently. They effectively prevented the epidemic from spreading outside this area of Gujarat through intensive field investigation and kept the death toll to a minimum given the potential for how extreme a large-scale epidemic of pneumonic plague could quickly have become.
This gives a brief, and somewhat crude, history of plague, which I think is enlightening for this disease. This is not only because it has afflicted the human species for a long time. Many diseases have done so. Malaria, tuberculosis, and many other of the major killers of today have afflicted our species for millennia. But plague is a little different. Or rather, it sits a little differently in our collective imagination. This is probably because it represents some of humanity's first experiences with pandemics. Perhaps our first recognition of ourselves as a "global" species, as the trade routes opened vast geographies to local populations, also came with the recognition that we could be rapidly wiped-out in large numbers by an unknown scourge for which no corner of our shared landscapes was safe. It seems to breed a unique cross-cultural fear that has been passed down in the recordings of many peoples across countless generations. We need look no further than 1994 and the panic that caused the movement of an entire local population in India and the brief collapse of one of the largest economies on earth. Such fear is completely unfounded given today's geographic contexts and pathogen ecologies, which will be described in detail below. But the fear, however unwarranted given plague's actual threat, undeniably lives in our memory.
So, on to the disease.
Plague is caused by a bacterium known as Yersinia pestis in the family Enterobacteriaceae. This is a gram-negative bacillus and is a facultative intracellular organism. Here is a picture of a group of Y. pestis:
Notice the strips of the rod-shaped bacillus structures.While this pathogen can be transmitted via open wounds or skin abrasion, and by lung secretions in the rare pneumonic form of plague, it is primarily transmitted as bubonic plague by another arthropod vector. In this case the vector is the rodent flea:
While there are several species of flea, Xenopsylla cheopis (common name, Oriental rat flea) is the most important vector for human transmission, though Nosopsyllus fasciatus (common name, Northern rat flea) can also spread disease to humans from the rodent reservior.
The mode of the flea vector transmission of Y. pestis is particularly interesting. Let's take a moment to discuss some of the important aspects of this arthropod, and then I will come back to its transmission of plague.
Fleas are quite fascinating creatures. They are very small, ranging from 1.5 to 3 mm in length. But they are superb jumpers. They can jump approximately 20 cm with respect to the vertical, and 30 cm in the horizontal plane, which means they can jump approximately 200 times the length of their body! As such, they are the second best jumpers in the whole of the known animal kingdom. They need this jumping capacity for locomotion because they are wingless and so do not fly, and they also need to be able to propel themselves through host hair or feathers. Fleas have extremely tough exoskeletons, which are capable of withstanding extemes of pressure (relative to their mass).
Here is an interesting video on the mechanics of the jumping flea produced by Discovery News:
The life cycle of the flea is unusual in that it is necessary to consider the organization and structure of an entire flea community in order to understand their ecology. The flea life cycle is comprised of four primary forms: egg, larva, pupa, and adult. While these stages are similar to other vectors we have discussed before (e.g. mosquitoes and sandflies), their population dynamics are quite different. Most notably are the weighting of the population by its individual life stages:
Few words are as emotive in the world of public health as the Plague. Indeed, it is unique among diseases in that the invocation of its very name demands not only the name itself, but also the definite article. So embedded in the depths of the human imagination, this malady requires all others to stand upon the order of its own coming and going. The Plague. Even the font seems to shift and contort into grotesque features as you read the written word upon the page. The fixed and fantastically dull, Times New Roman, is transformed before your eyes into something only the fear of the memory of countless generations can transcribe:
The Plague.
But enough of hyperbole, why should this disease evoke such profound dread? First and foremost, it does not constitute a large-scale public health burden, or threat, so why the fuss? HIV/AIDS, tuberculosis, and malaria are the big three, which account for miillions of lives lost and immeasurable morbidity each year around the world. None of these, not even HIV as we currently live through its devastating pandemic, invoke the visceral response of plague. The loathsome neglected tropical diseases such as trypanosomiasis and leishmaniasis, which constitute large burdens across the developing world, can inflict horrible morbidity and mortality that devastates communities, but even these typically are not feared like the plague. The yearly varied influenza virus in all its forms, constantly renewing itself each season, does not even motivate most to vaccinate! The emergence of the superbugs, those virulent bacteria that have developed resistance to antibiotic therapy such as methicillin-resistant staphylococcus aureus, may instill an unequaled fear among infectious disease clinicians (quite justified, I might add), but they do not, generally, strike fear into the hearts of the general populace. Indeed, "plague" has such powerful connotation, that it has come to be used to refer to any severe epidemic of infectious disease.
So, wherefore this dread? Likely it comes down through our shared historical experience as human beings, and what was probably one of the first instances of a human pandemic (other early pandemics were probably due to smallpox). In fact, historically, there have been three plague pandemics. The First plague pandemic occurred from 541 to 542 AD and is known as the Justinian Plague, so called because it ravaged the Byzantine Empire under the rule of the emperor Justinian. Geographically speaking, it affected the Mediterranean, including southern Europe, northern Africa, and the near-Middle East, shown here in red:
Key to the emergence of this, perhaps first, pandemic disease (though, in reality this was probably second to a smallpox pandemic during the preceding Roman empire), were the extensive trade routes across very large geographic landscapes. As people traversed large geographic spaces by land and sea, they brought their trade goods with them...and their rats...and their rats' fleas. And so the widespread dissemination of plague was facilitated by trade.
The Second plague pandemic raged from 1347 to 1351 and is known as the "Black Death". It originated in central Asia and spread rapidly following the Silk Road: once again the pandemic emerged by way of the movement of people and their rodents along the routes of trade. The spatial-temporal spread of the Second pandemic is detailed nicely in this animated graphic:
This pandemic was truly devastating. It is believed that more than 100 million people died from this plague, which represented almost 25% of the global human population. No pandemic since has claimed the lives of such a large proportion of the human population. In addition, this pandemic affected very large areas, particularly China and Europe, where the infection killed about 1/2 and 1/3 of the populations, respectively. In these regions the incidence of pneumonic plague became relatively high as the infection became more established in humans. As such, human-to-human transmission became much more prominent, which is not typically how humans acquire the infection. It is worth restating the mortality above: this pandemic killed 1/2 the population of China and 1/3 the population of Europe. It also killed 1/8 the population of Africa. The scope of such a catastrophe is utterly incomprehensible to the modern world. All we can do is stare in amazement at the staggering statistics.
Perhaps with an historical perspective, we can start to get a sense of why this disease has been so feared throughout the human experience.
We are currently living through the Third plague pandemic. This began in 1855 in China and has killed more than 12 million people there and in India over the last one and a half centuries. This pandemic has spread to each continent with permanent human populations (i.e. excluding Antarctica). It was widely distributed as bubonic plague by shipping routes, with the domestic rats carried aboard shipping vessels transporting the plague bacteria to ports all around the world. Once again, plague followed the trade routes. East, Southeast and South Asia were widely affected up until the turn of the 19th century. The plague reached the United States in 1900 and accounted for urban plague outbreaks up until the 1920s. As described in detail below, currently the world's largest remaining sylvan focus of plague continues to exist in the western and southwestern US. Though most of the human cases do not come from there. Today the largest number of human cases (over 99%) come from sub-Saharan African countries. The Democratic Republic of Congo and Madagascar both see a particularly large number of cases, relatively speaking, as it is still a rare disease. One of the most striking demonstrations of the popular fear of plague that remains into modern times comes from Surat, India. In 1994 an outbreak of pneumonic plague resulted in 54 deaths in the town of Surat in Gujarat, India. During this outbreak, a staggering 300,000 people fled the area as the news of epidemic broke. This massive popular migration brought the local economy and social infrastructure to a standstill and created a humanitarian disaster. The epidemic also affected the larger national Indian economy because of the response of the international community and the subsequent effect on India's industries. Nevertheless, the Indian public health authorities should be applauded for controlling the situation quickly and efficiently. They effectively prevented the epidemic from spreading outside this area of Gujarat through intensive field investigation and kept the death toll to a minimum given the potential for how extreme a large-scale epidemic of pneumonic plague could quickly have become.
This gives a brief, and somewhat crude, history of plague, which I think is enlightening for this disease. This is not only because it has afflicted the human species for a long time. Many diseases have done so. Malaria, tuberculosis, and many other of the major killers of today have afflicted our species for millennia. But plague is a little different. Or rather, it sits a little differently in our collective imagination. This is probably because it represents some of humanity's first experiences with pandemics. Perhaps our first recognition of ourselves as a "global" species, as the trade routes opened vast geographies to local populations, also came with the recognition that we could be rapidly wiped-out in large numbers by an unknown scourge for which no corner of our shared landscapes was safe. It seems to breed a unique cross-cultural fear that has been passed down in the recordings of many peoples across countless generations. We need look no further than 1994 and the panic that caused the movement of an entire local population in India and the brief collapse of one of the largest economies on earth. Such fear is completely unfounded given today's geographic contexts and pathogen ecologies, which will be described in detail below. But the fear, however unwarranted given plague's actual threat, undeniably lives in our memory.
So, on to the disease.
Plague is caused by a bacterium known as Yersinia pestis in the family Enterobacteriaceae. This is a gram-negative bacillus and is a facultative intracellular organism. Here is a picture of a group of Y. pestis:
Notice the strips of the rod-shaped bacillus structures.While this pathogen can be transmitted via open wounds or skin abrasion, and by lung secretions in the rare pneumonic form of plague, it is primarily transmitted as bubonic plague by another arthropod vector. In this case the vector is the rodent flea:
While there are several species of flea, Xenopsylla cheopis (common name, Oriental rat flea) is the most important vector for human transmission, though Nosopsyllus fasciatus (common name, Northern rat flea) can also spread disease to humans from the rodent reservior.
The mode of the flea vector transmission of Y. pestis is particularly interesting. Let's take a moment to discuss some of the important aspects of this arthropod, and then I will come back to its transmission of plague.
Fleas are quite fascinating creatures. They are very small, ranging from 1.5 to 3 mm in length. But they are superb jumpers. They can jump approximately 20 cm with respect to the vertical, and 30 cm in the horizontal plane, which means they can jump approximately 200 times the length of their body! As such, they are the second best jumpers in the whole of the known animal kingdom. They need this jumping capacity for locomotion because they are wingless and so do not fly, and they also need to be able to propel themselves through host hair or feathers. Fleas have extremely tough exoskeletons, which are capable of withstanding extemes of pressure (relative to their mass).
Here is an interesting video on the mechanics of the jumping flea produced by Discovery News:
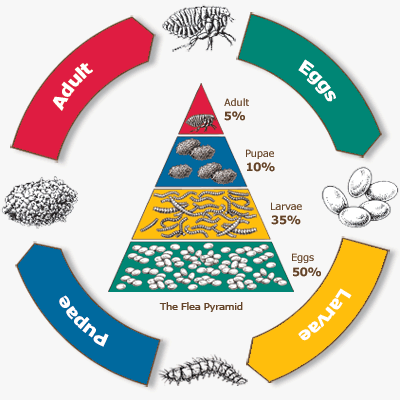
The life cycle of the flea is unusual in that it is necessary to consider the organization and structure of an entire flea community in order to understand their ecology. The flea life cycle is comprised of four primary forms: egg, larva, pupa, and adult. While these stages are similar to other vectors we have discussed before (e.g. mosquitoes and sandflies), their population dynamics are quite different. Most notably are the weighting of the population by its individual life stages:
In the graph above notice a 10-fold difference in the proportions of adults and eggs in the flea community. Female fleas are prodigious egg producers, laying up to 50 eggs per day, and they do so directly on the host itself. The eggs are only loosely attached to the surface of the host, typically in hair or feathers, or on the skin. Since the eggs do not adhere to the host they easily fall off, usually becoming deposited where that hosts rests or sleeps. When the eggs hatch the larvae will withdraw to crevices, cracks, nooks and crannies, but nevertheles, remaining in close proximity to where the host rests. The larvae then begin to form a cocoon around them known as the puparium, which will serve as the protective shell in which the pupae develop before emerging as fully formed adults. Critical to this life cycle is that through each stage of development the flea remains close to the host, or, at least, to the host's resting place. Adults emerge from their cocoon when one or more specific signals is identified by the cocooned adult: the adult flea senses the host's movement; the flea senses pressure from the superposition of the host's body weight (thus the flea's need for a very hard body, which is highly resistant to pressure); the flea senses the body heat of its host; or the flea senses carbon dioxide released as its host breathes. Any of these signals can trigger the emergence of the fully developed flea from its puparium. However, until the flea recieves such signals, it will remain in the cocoon. It can survive for months in this state, without feeding, waiting out its host's return. Here is the general life cycle of the flea in a nice graph produced by the Centers for Disease Control and Prevention (CDC):
And here is a short video produced by the Blue Springs Animal Hospital and Pet Resort detailing the different stages of the flea life cycle:
So, returning to the flea as a vector for plague, we are left with the question: how do fleas transmit Y. pestis? It is a unique process, indeed. In fact, unlike most of the arthropod vector-pathogen relationships we have explored so far, the relationship between the flea and Y. pestis is NOT commensal, it is antagonistic to the flea. For example, the Anopheles and Aedes mosquitoes, which transmit the Plasmodium parasites and dengue virus, respectively, appear to be commensal with those pathogens. This means the pathogens do their host vectors neither harm nor good at the population level of the vector. They do harm once they are transmitted to the human host, but, on average, not to the vector. Fleas, on the other hand, are directly harmed by the plague bacillus, and, crucially, it is by this very mechanism of antagonism that Y. pestis can be transmitted to new mammalian hosts! Here's how it works:
When fleas take a blood meal from a host infected with Y. pestis, some of the fleas will in fact clear the infection altogether and will not be able to infect a new host. But other fleas in the population will become infected. In these infected fleas, the plague bacilli multiply in the midgut of the flea. After about 2 days, the Y. pestis colony begins to form a biofilm within the stomach, esophagus, and proventriculus, which is the sphincter that separates the esophagus and stomach of the flea. This biofilm continues to grow in subsequent days impeding the passage of blood from the esophagus to the stomach. At some point between approximately 3 and 9 days after the flea's initial infection the proventriculus will become completely blocked, and thus allows no passage of blood to the stomach. The flea repeatedly tries to feed but cannot pass the blood from the esophagus to the stomach due to the blocked gut tract. Instead, the host's blood mixes with Y. pestis in the esophagus of the flea and is regurgitated back into the open bite wound of the host, causing a new infection if the host is susceptible (i.e. not already infected). And the flea eventually dies of starvation. So Y. pestis is really no one's friend. You may think it a cruel world, but biology works and so there you have it.
The primary reservoir hosts for Y. pestis are rodents. Rodents and their fleas maintain the infection cycle in both sylvan and domestic rodents. This is very important for human transmission, as plague is essentially a zoonotic disease. Y. pestis is first and foremost a sylvan pathogen, with wild rodents acting as the primary reservoir in nature. However, as is so often the case in human disease ecology, anthropogenic manipulation of the environment first creates, then brings humans into contact with, fragmented landscapes. In the case of plague, it is not simply human encroachment, but the vermin (i.e. domestic rodents) humans bring with them. As domestic rodents share landscapes with sylvan rodents in peri-domestic ecotones that bridge natural habitat with agricultural or urban communities, so too do these rodents share the Y. pestis pathogen, thus maintaining a dual sylvan-domestic reservoir cycle. In settings of poverty, overcrowding, and poor hygiene, humans often share close quarters with their domestic rodents and thus become exposed to the rodent fleas that carry Y. pestis. These critical features of 1) the maintenance of the sylvan-domestic reservoir, and 2) the close proximity of humans to domestic rodents in conditions of poverty, have largely defined the disease ecology in local epidemics where Y. pestis is endemic in rodent populations. There are other critical factors for the emergence of large epidemics or pandemics, like the large-scale transportation of people and goods over trade routes (discussed above) or the transition from bubonic plague to pneumonic plague (discussed below), but the basic rodent disease ecology is fundamental for establishing human transmission.
Let's turn now to a discussion of the disease.
There are three distinct clinical manifestations of plague. These three are bubonic, septicemic and pneumonic plague. When humans are infected with Y. pestis, the most common manifestation is the first form, bubonic plague. This is characterized by general malaise, fever, chills, myalgia and sometimes seizures. As you can infer, these symtpoms are fairly indistinct and so initial diagnosis can easily be missed if plague is not expected and if the signature characteristic is missing: the swelling of the bubos:
In bubonic plague Y. pestis invades the lymphatic system. In particular, this bacterium is able to evade the phago-lysosomal enzymatic activity of the host's macrophages (similar to the Leishmania parasites, you will recall). Once established in the lymph nodes, the bacteria multiply and cause localized swelling in the lymph nodes, which can eventually become necrotic. The lymph nodes most often involved are in the groin area, but any lymph nodes can be involved. The term bubo is derived from the Greek description of these swollen lymph nodes. The English word "booboo", used as a generic description of any sore or injury for children, comes from this very early Greek description of one of the signs of bubonic plague. Case-fatality from untreated bubonic plague ranges between 30% and 60%, according to the World Health Organization (WHO), and between 50% and 60% according to the Control of Communicable Diseases Manual, which is produced by the American Public Health Association.
Bubonic plague symptoms:
Septicemic plague is extremely severe and, if untreated, will almost always result in death. This form of plague occurs when the bateria enter the blood and begin to multiply here rather than in the lymph. Remember that Y. pestis is gram-negative, which means it is an important producer of endotoxins. In the case of this particular pathogen, the endotoxins are responsible for a disseminated intravascular coagulation when the bacteria enter the blood, which causes micro-clotting, especially in the periphery. This can result in necrosis in peripheral tissues with associated gangrenous hands and feet:
Because clotting factors become depleted, this coagulopathy simultaneously diminishes the host's ability to control bleeding, which results in hemorrhaging under the skin or in other organs such as the lungs. Death from speticemic plague can be extraordinarily rapid. Treatment is required within 24 hours of infection or the associated case-fatality is very close to 100%.
Pneumonic plague is a Y. pestis infection that involves the lungs. Like septicemic plague, pneumonic plague is much more virulent than bubonic plague. But unlike bubonic and septicemic plague, pneumonic plague is extrememly contagious directly between humans via airborne transmission. Most cases of pneumonic plague in humans are secondary to bubonic plague, which, as described above, is primarily transmitted via the flea vector. However, once a human host develops pneumonic plague this person is capable of transmitting the infection through lung secretions, as droplets and droplet nuclei, that can spread through coughing and talking. When these aerosilized emissions are inhaled new infection occurs, resulting in primary pneumonic plague. While this mode of transmission is rare, in an epidemic it can play an important role in the rapid movement of the disease through populations. Indeed, pneumonic plague likely played an important role in the 14th century pandemic that caused the Black Death.
Pneumonic plague symptoms:
Untreated, pneumonic plague has a case-fatality similar to that of septicemic plague: very close to 100%.
In order to understand the epidemiology of plague we need to examine the geographic distribution of enzootic disease. By enzootic disease, we mean the the level of baseline, prevalent disease that is typically present in a single-animal or mixed-animal population. Enzootic disease is punctuated by epizootic disease, which refers to an increase in the number of animal infections above the normal baseline level of infection. These terms describe disease in animal populations and are analogous to the terms endemic and epidemic that we use to describe disease in human populations. Disease cannot be transmitted to humans in areas where there are no enzootic foci of infection because, as described above, Y. pestis is first and foremost a sylvan rodent pathogen. The following map, produced by the WHO, shows the geographic distribution of plague. Countries reporting plague are colored orange, whereas the enzootic foci within countries reporting plague are colored red:
The country with the largest enzootic foci of plague is the United States. Most cases of human plague in the US are acquired in rural settings, transmitted by rodent flea vectors, and present as bubonic plague. There has not been any urban plague in the US since the 1924-25 epidemic in Los Angeles. According to the CDC, there are about a dozen plague cases per year in the US, and anywhere between 1000 and 3000 cases per year worldwide. Therefore, even though the US has by far the largest enzootic foci of Y. pestis of any country or larger geographic area in the world, it contributes relatively few human cases of plague each year. Most of the human cases of plague throughout the 20th century came from central Asia, China, and South Asia, as well as Southeast Asia during the Vietnam war, but currently most humans cases of plague are coming from sub-Saharan Africa and, to a lesser extent, Mongolia:
The Democratic Republic of Congo contributes more than 1000 cases per year to the global total, making this the country with the most active foci for human plague in the world. The long term wars that have devastated this country, contributing to massive population movements and extremes of poverty, have probably greatly contributed to this active plague foci.
The peridomestic transmission cycle in rodents is the primary source of threat for human transmission in rual areas of Africa and South and Central Asia today. In these areas, relatively high rural population densities combine with the poor hygiene and crowding associated with poverty, which in turn conspire with the sharing of infected fleas between sylvan and domestic rodents. Thus, the cycle of the dreaded plague is maintained.