Tweet
Typhus consists of three different disease types, all of which are vectored by arthropods. There is epidemic typhus, murine typhus and scrub typhus. None of these should be confused with typhoid fever, which is a fecal-orally transmitted, often water- or food-borne, infection caused by the bacterium Salmonella enterica serovar Typhi, often simply referred to as Salmonella Typhi. Typhus is very different in all respects: infectivity, pathogenicity, clinical manifestation, transmission. etc. So, don't make the common mistake of confusing typhus and typhoid fever.
We will examine each of the three forms of typhus separately.
EPIDEMIC TYPHUS. This is the "human" form of disease. I have human in quotes because humans are indeed susceptible to all three forms of typhus, but humans serve as the reservoir for epidemic typhus. However, this may not be entirely true either as evidence is accruing that shows flying squirrels may comprise an additional reservoir for epidemic typhus in North America. Nevertheless, this secondary reservoir does not constitute a significant source of human disease.
Epidemic typhus is caused by Rickettsia prowazekii. You can see these organisms below as the dark, rod-like structures:
R. prowazekii is a gram-negative, obligate intracellular aerobic α-proteobacterium in the family Rickettsiaceae and the order Rickettsiales. This is a definitive rickettsial disease, and it can be quite dangerous as we will see.
R. prowazekii requires an arthropod vector to infect the human host. In this case, the vector is the body louse, Pediculus humanus humanus (more commonly known as Pediculus humanus corporis):
Unlike head lice and pubic lice, body lice typically live in clothing rather than on the host. Body lice have a fairly straightforward life cycle as depicted here in this graph by the Centers for Disease Control and Prevention (CDC):
While this is an adequate representation of the required stages that body lice must complete it is important to note that the rectangle box with the life stages is specific to head lice (Pediculus humanus capitas), rather than body lice. So, in this depiction, the head lice nit is attached to a shaft of hair. Body lice will sometimes attach their eggs to body hair in this same way, but their eggs are much more commonly found in the seams and/or folds and creases of clothing and bedding. In fact, body lice are typically only found on the body when taking a blood meal.
Transmission of R. prowazekii humans occurs similarly to Chagas disease, i.e. by introduction of feces into the bite wound. The typhus pathogen remains in the gut of the body louse when the louse takes its blood meal from an infected human. Once infected in this way the louse can subsequently infect new human hosts upon the taking of subsequent blood meals. The Rickettsia are deposited in the louse feces on the skin of its host while feeding. Rubbing or scratching the bite wound of the louse introduces the bacteria into the human host and initiates an infection. It is also important to note that R. prowazekii can remain viable in dried louse feces for several days after defecation, and, in fact, one strain can remain infective for months. Because the pathogen can remain infective in dried louse feces, it is possible for this pathogen to aerosolize and transmit via the air-borne route. This mode of transmission, however, is not common.
R. prowazekii, like almost all Rickettsia, infect the endothelial cells in humans. There they replicate in the cytoplasm of the cell and subsequently disseminate throughout the host by the blood circulation. The Rickettsia induce vascular damage by way of their cytopathic effect on the endothelium, as well as by stimulating immune-effector cells, which may also have some deleterious effect on the vasculature even as they orchestrate clearance of the bacteria.
Clinically apparent, acute infection is usually abrupt in humans. Epidemic typhus typically presents with rapid-onset of headache, myalgia, rash, hypotension, mental confusion, sensitivity to light, coughing, chills, and an ongoing high fever. The rash first appears as a macular errupution on the chest usually about five days after the appearance of fever and initial symptoms. From the chest the rash spreads to the rest of the trunk and the periphery extending down the legs and arms, but usually excluding the plantar and palmar surfaces and the face:
If the person survives the infection, symptoms abate just as rapidly as the onset, with a break in fever that typically occurs roughly two weeks after the onset of clinically apparent disease. Untreated, the case-fatality associated with epidemic typhus ranges from 10% to 40%, so outbreaks are of considerable concern. This disease is reportable to the WHO if an outbreak is believed to have the potential for a widespread epidemic.
The landscape of epidemic typhus is, in large part, defined by four important factors, two of which are physical and the other two, social. One aspect of the physical landscape that ultimately determines the occurrence of epidemic typhus is water. The availability of water is crucial because this decides whether people, at the population level, bathe and launder clothes on a regular basis. Temperature is another critical attribute of the physical landscape. Places that are high in latitude or altitude tend to be cold places. Cold temperatures, often combined with low availability of water, promote the continuous wearing of heavy clothing, which is ideal habitat for the body louse. The other two social factors are residential overcrowding and poor hygiene. The latter is obviously very strongly associated with the availability of water, but both social factors are also conditions of povery. So epidemic typhus can largely be delineated across physical and social space by determining the availability of water and the average temperature in connection with the population density and the level of poverty.
Circumstances that force people into these settings, such as war and natrual disasters, are very important risk contexts for epidemic typhus.
MURINE TYPHUS (Also known as endemic typhus). This form of typhus is caused by Rickettsia typhi (and sometimes by R. felis), and its reservoir is rodents (the reservoir hosts for R. felis are cats and opossums). This pathogen is vectored by the rat flea (Xenopsylla cheopis), which, as you may remember from our discussion of plague, is quite a fascinating arthropod. Here is a recap of the flea if you do not remember:
The clincal presentation of murine typhus matches that of epidemic typhus, with the excpetion that the clinical course is typically much milder in murine typhus. The case-fatality in untreated murine typhus is less than 1%, however it does increase in older age.
SCRUB TYPHUS. This form of typhus is caused by Orientia tsutsugamushi. The epidemiology of scrub typhus in humans is unique in that the reservoir and the vector are the same organism: the mite, or more specifically. trombiculid (harvest) mites. In particular we are talking about a specific family of mites known as the Trombiculidae, which, along with ticks, are Acari arachnid arthropods. There are several different genera and species of "harvest mite", most of which do not vector infections. However, one species does act as a vector for human disease throughout much of Asia and the South Pacific: Leptotrombidium deliense. L deliense is both the reservoir for O. tsutsugamushi and the vector for human transmission.
Let's take a closer look at the life cycle of this mite. The length of the life cycle of most Trombiculidae can vary dramatically, ranging from only a couple months or less to one year. Of course, there are differences in life cycle duration across different species, but duration is also largely determined by temperature and climate. In temperate regions of the globe, these mites typically require long periods to complete the life cycle, while in tropical climates the life cycle duration is usually much shorter. The length of life cycle duration also, not surprisingly, correlates with the number of mite generations that will emerge each season in a given mite population. Shorter life cycle duration allows for a larger number of cycles and thus more generations of mites per season. Here is a simple depiction of the four life cycle stages of trombiculid mites:
In temperate regions, adult females lay their eggs in ground vegetation in the Spring season once soils reach a specific temperature. After about a week, pre-larvae emerge from the eggs, but are as yet not fully formed. They require roughly one more week to develop their fourth leg, since they come into the world with only three. When they are fully developed larvae they are ready to feed on their host. These larvae are often referred to as "chiggers", which is a misnomer that comes from the chigoe flea. Interestingly, the larva stage of the mite life cycle is the only stage that is an ectoparasite. As nymphs and adults, the mites are not parasitic. The larvae typically await a passing host collectively, by agregating together in bunches in understory vegetation, including ground-based fallen vegetation and scrub and bush plants. These mite larvae are reddish in color, covered in hairs and very, very small. Here is a picture:
Since O. tsutsugamushi is transmitted transovarially between the adult female mite and her eggs, the larvae need not encounter an infected host to become infected. If the parent female was infected, the larvae will emerge infected and, upon reaching their first host, can immediately pass on the infection.
In some symptoms, the clinical presentation of scrub typhus is similar to that of epidemic and murine typhus, but it has a unique presenation as well. Headache, myaglia, sustained fever and a macular rash are similar symtpoms that scrub typhus shares with the other typhus forms. Its other symtpoms, however, diverge. To begin, scrub typhus typically presents with an initial eschar, which is an ulcer on the skin surface at the point of attachment for the mite larva ("chigger") that appears well after the larva has dropped off. Here is an example of a scrub typhus eschar:
Within days of the appearance of the eschar, other symptoms, such as headache, body ache, fever and sweating set in. Lymphadenopathy and splenomegaly may also begin to appear at this stage. Abdominal pain is also common in scrub typhus. Following, the maculopapular rash appears on the trunk and extends to the extremities, similar to the other typhus forms. Another unique feature is that scrub typhus can be hemorrhagic, with bleeding and coagulation pathologies present. Other complications include pneumonitis, encephalitis and myocarditis, all of which, if they do occur, present later in the disease course. The case-fatality associated with untreated scrub typhus varies dramatically across geography and pathogen strain, but can exhibit the highest associated mortality of all the typhus forms (range 1% to 60%). Older age is again associated with greater case-fatality.
The landscape epidemiology of scrub typhus is extraordinarily precise. Indeed, scrub typhus may be perhaps the most hyper-locally determined disease humans expereince. The overall global distribution forms the scrub typhus "triangle", wherein the northern boundary is Siberian Russia, the eastern boudary includes all of east and southeast Asia and extends well into the South Pacific islands, the southern boundary is northern Australia, and the western boundary extends beyond Afghanistan into central Asia. Here is a map of this global distribution:
But, as mentioned above, this is a disease defined by micro-geography, so any global geographic perspective of a scrub typhus "triangle" provides very little scope to help us epidemiologically delineate the transimission landscape of this disease.
First off, it is important to keep in mind that scrub typhus is a disease whose reservoir is the mite, but which can also act as a zoonotic disease by affecting rodents in a sylvan cycle of disease that commonly occurs in parallel with its natural reservoir. Human infection is accidental, and so, too, is infection in rodents. Nevertheless, the existence of a zoonotic sylvan cycle can amplify transmission in a given mite population.
Small communities of infected mite populations intersect with rodent populations to form small tsutsugamushi islands across the landscape. Indeed, some of these isolated infection zones can be only one meter square in size. These scrub typhus islands are detremined by the relevant reservoir mite populations, which in turn are shaped by precise fluctuations in soils, ground vegetation cover, temperature and humidity, and precipitation. Too moist, and the mites do not like the area. Too dry, and ground vegetation may be too sparse to support mite populations. Thus, extreme precision is required for transmission to humans. While these transmission islands define a high degree of landscape specificity for the occurrence of scrub typhus, they are not necessarily rare across the landscape depending on where in the global "triangle" they are located. Thus, this unique transmission potential has resulted in wide-spread epidemics under conditions of human encroachment on natural habitat characterized by overgrown vegetation. Particularly senstitive areas include forest and ecotones at marginally deforested locations and forrest clearings, as well as agricultural irrigation projects in arid or semi-arid regions. Workers in these settings and communities establishing new settlements tend to be at highest risk. Also, military conflict in these endemic landscapes, have been associated with very large outbreaks of scrub typhus in humans. This was a significant problem during World War II military campaigns in southeast Asia, and the South Pacific. In this setting, transmission to humans can be so extensive that as many as 50% of combatants are infected.
A more realistic global map of scrub typhus might look something like this map below, which was published in A Manual of Tropical Medicine in 1964. Needless to say, given that this is now 50 years old it may no longer be relevant, but it does highlight areas wherein the isolated islands of scrub typhus transmission might be commonly encountered:
Field surveys undertaking the remapping of scrub typhus across the traditional geographic "triangle" would be a very useful public health endeavor for the 21st century given the rapidly changing structures of populations across much of Asia and the concurrent escalation of human encroachment on natural habitat.
Typhus consists of three different disease types, all of which are vectored by arthropods. There is epidemic typhus, murine typhus and scrub typhus. None of these should be confused with typhoid fever, which is a fecal-orally transmitted, often water- or food-borne, infection caused by the bacterium Salmonella enterica serovar Typhi, often simply referred to as Salmonella Typhi. Typhus is very different in all respects: infectivity, pathogenicity, clinical manifestation, transmission. etc. So, don't make the common mistake of confusing typhus and typhoid fever.
We will examine each of the three forms of typhus separately.
EPIDEMIC TYPHUS. This is the "human" form of disease. I have human in quotes because humans are indeed susceptible to all three forms of typhus, but humans serve as the reservoir for epidemic typhus. However, this may not be entirely true either as evidence is accruing that shows flying squirrels may comprise an additional reservoir for epidemic typhus in North America. Nevertheless, this secondary reservoir does not constitute a significant source of human disease.
Epidemic typhus is caused by Rickettsia prowazekii. You can see these organisms below as the dark, rod-like structures:
R. prowazekii is a gram-negative, obligate intracellular aerobic α-proteobacterium in the family Rickettsiaceae and the order Rickettsiales. This is a definitive rickettsial disease, and it can be quite dangerous as we will see.
R. prowazekii requires an arthropod vector to infect the human host. In this case, the vector is the body louse, Pediculus humanus humanus (more commonly known as Pediculus humanus corporis):
Unlike head lice and pubic lice, body lice typically live in clothing rather than on the host. Body lice have a fairly straightforward life cycle as depicted here in this graph by the Centers for Disease Control and Prevention (CDC):
While this is an adequate representation of the required stages that body lice must complete it is important to note that the rectangle box with the life stages is specific to head lice (Pediculus humanus capitas), rather than body lice. So, in this depiction, the head lice nit is attached to a shaft of hair. Body lice will sometimes attach their eggs to body hair in this same way, but their eggs are much more commonly found in the seams and/or folds and creases of clothing and bedding. In fact, body lice are typically only found on the body when taking a blood meal.
Transmission of R. prowazekii humans occurs similarly to Chagas disease, i.e. by introduction of feces into the bite wound. The typhus pathogen remains in the gut of the body louse when the louse takes its blood meal from an infected human. Once infected in this way the louse can subsequently infect new human hosts upon the taking of subsequent blood meals. The Rickettsia are deposited in the louse feces on the skin of its host while feeding. Rubbing or scratching the bite wound of the louse introduces the bacteria into the human host and initiates an infection. It is also important to note that R. prowazekii can remain viable in dried louse feces for several days after defecation, and, in fact, one strain can remain infective for months. Because the pathogen can remain infective in dried louse feces, it is possible for this pathogen to aerosolize and transmit via the air-borne route. This mode of transmission, however, is not common.
R. prowazekii, like almost all Rickettsia, infect the endothelial cells in humans. There they replicate in the cytoplasm of the cell and subsequently disseminate throughout the host by the blood circulation. The Rickettsia induce vascular damage by way of their cytopathic effect on the endothelium, as well as by stimulating immune-effector cells, which may also have some deleterious effect on the vasculature even as they orchestrate clearance of the bacteria.
Clinically apparent, acute infection is usually abrupt in humans. Epidemic typhus typically presents with rapid-onset of headache, myalgia, rash, hypotension, mental confusion, sensitivity to light, coughing, chills, and an ongoing high fever. The rash first appears as a macular errupution on the chest usually about five days after the appearance of fever and initial symptoms. From the chest the rash spreads to the rest of the trunk and the periphery extending down the legs and arms, but usually excluding the plantar and palmar surfaces and the face:
If the person survives the infection, symptoms abate just as rapidly as the onset, with a break in fever that typically occurs roughly two weeks after the onset of clinically apparent disease. Untreated, the case-fatality associated with epidemic typhus ranges from 10% to 40%, so outbreaks are of considerable concern. This disease is reportable to the WHO if an outbreak is believed to have the potential for a widespread epidemic.
The landscape of epidemic typhus is, in large part, defined by four important factors, two of which are physical and the other two, social. One aspect of the physical landscape that ultimately determines the occurrence of epidemic typhus is water. The availability of water is crucial because this decides whether people, at the population level, bathe and launder clothes on a regular basis. Temperature is another critical attribute of the physical landscape. Places that are high in latitude or altitude tend to be cold places. Cold temperatures, often combined with low availability of water, promote the continuous wearing of heavy clothing, which is ideal habitat for the body louse. The other two social factors are residential overcrowding and poor hygiene. The latter is obviously very strongly associated with the availability of water, but both social factors are also conditions of povery. So epidemic typhus can largely be delineated across physical and social space by determining the availability of water and the average temperature in connection with the population density and the level of poverty.
Circumstances that force people into these settings, such as war and natrual disasters, are very important risk contexts for epidemic typhus.
MURINE TYPHUS (Also known as endemic typhus). This form of typhus is caused by Rickettsia typhi (and sometimes by R. felis), and its reservoir is rodents (the reservoir hosts for R. felis are cats and opossums). This pathogen is vectored by the rat flea (Xenopsylla cheopis), which, as you may remember from our discussion of plague, is quite a fascinating arthropod. Here is a recap of the flea if you do not remember:
Fleas are quite fascinating creatures. They are very small, ranging from 1.5 to 3 mm in length. But they are superb jumpers. They can jump approximately 20 cm with respect to the vertical, and 30 cm in the horizontal plane, which means they can jump approximately 200 times the length of their body! As such, they are the second best jumpers in the whole of the known animal kingdom. They need this jumping capacity for locomotion because they are wingless and so do not fly, and they also need to be able to propel themselves through host hair or feathers. Fleas have extremely tough exoskeletons, which are capable of withstanding extemes of pressure (relative to their mass).
Here is an interesting video on the mechanics of the jumping flea produced by Discovery News:
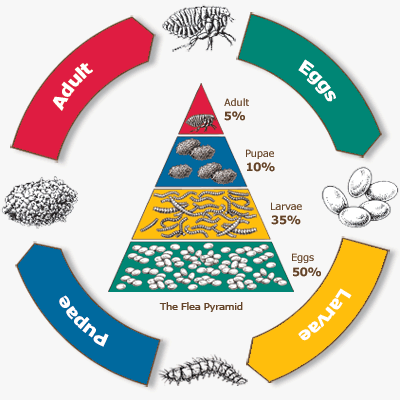
The life cycle of the flea is unusual in that it is necessary to consider the organization and structure of an entire flea community in order to understand their ecology. The flea life cycle is comprised of four primary forms: egg, larva, pupa, and adult. While these stages are similar to other vectors we have discussed before (e.g. mosquitoes and sandflies), their population dynamics are quite different. Most notably are the weighting of the population by its individual life stages:
In the graph above notice a 10-fold difference in the proportions of adults and eggs in the flea community. Female fleas are prodigious egg producers, laying up to 50 eggs per day, and they do so directly on the host itself. The eggs are only loosely attached to the surface of the host, typically in hair or feathers, or on the skin. Since the eggs do not adhere to the host they easily fall off, usually becoming deposited where that hosts rests or sleeps. When the eggs hatch the larvae will withdraw to crevices, cracks, nooks and crannies, but nevertheles, remaining in close proximity to where the host rests. The larvae then begin to form a cocoon around them known as the puparium, which will serve as the protective shell in which the pupae develop before emerging as fully formed adults. Critical to this life cycle is that through each stage of development the flea remains close to the host, or, at least, to the host's resting place. Adults emerge from their cocoon when one or more specific signals is identified by the cocooned adult: the adult flea senses the host's movement; the flea senses pressure from the superposition of the host's body weight (thus the flea's need for a very hard body, which is highly resistant to pressure); the flea senses the body heat of its host; or the flea senses carbon dioxide released as its host breathes. Any of these signals can trigger the emergence of the fully developed flea from its puparium. However, until the flea recieves such signals, it will remain in the cocoon. It can survive for months in this state, without feeding, waiting out its host's return. Here is the general life cycle of the flea in a nice graph produced by the Centers for Disease Control and Prevention (CDC):
And here is a short video produced by the Blue Springs Animal Hospital and Pet Resort detailing the different stages of the flea life cycle:
So fleas maintain the endemicity in the primary rodent reservoir. However, unlike with the plague where Y. pesits was harmful to the flea, in murine typhus R. typhi does not harm the flea. In fact, the differences in the pathogenicity of these two bacteria in the flea reflect imporant differences in how the organisms are transmitted to human hosts. Recall that Y. pestis essentailly blocks the gut tract causing the flea to regurgitate its blood meal. As it does so, it regurgitates Y. pestis back into the bite wound and infects the human host. The flea is also ultimately killed because it can no longer feed. R. typhi, on the other hand, lives in the gut tract of the flea without harming it. The relatively healthy flea, infected with R. typhi, defecates while taking its blood meal. These feces contain R. typhi and are depostied on the skin of the host. As the host scratches the itchy bite wound, the contaminated feces are rubbed into the wound and thus infection of a new rodent, or human, is initiated. So, you can see this is the same mode of transmission utilized by R. prowazekii in its transmission of epidemic typhus via the louse vector. The difference is that R. prowazekii ultimately kills the louse vector, whereas R. typhi does not kill the flea vector.
Close contact between humans and rodents is again crucial to the epidemiology of murine typhus, as it is to the epidemiology of plague. The global distribution among humans, however, is far more widespread than plague:
Seroprevalence surveys have identified antibody evidence of murine typhus infection in over 10 percent of some populations in the southwestern United States, for example. The higher occurence of this rat flea-vectored disease in humans, relative to plague, may be due to the lack of a sylvan dependent infection cycle for murine typhus.
SCRUB TYPHUS. This form of typhus is caused by Orientia tsutsugamushi. The epidemiology of scrub typhus in humans is unique in that the reservoir and the vector are the same organism: the mite, or more specifically. trombiculid (harvest) mites. In particular we are talking about a specific family of mites known as the Trombiculidae, which, along with ticks, are Acari arachnid arthropods. There are several different genera and species of "harvest mite", most of which do not vector infections. However, one species does act as a vector for human disease throughout much of Asia and the South Pacific: Leptotrombidium deliense. L deliense is both the reservoir for O. tsutsugamushi and the vector for human transmission.
Let's take a closer look at the life cycle of this mite. The length of the life cycle of most Trombiculidae can vary dramatically, ranging from only a couple months or less to one year. Of course, there are differences in life cycle duration across different species, but duration is also largely determined by temperature and climate. In temperate regions of the globe, these mites typically require long periods to complete the life cycle, while in tropical climates the life cycle duration is usually much shorter. The length of life cycle duration also, not surprisingly, correlates with the number of mite generations that will emerge each season in a given mite population. Shorter life cycle duration allows for a larger number of cycles and thus more generations of mites per season. Here is a simple depiction of the four life cycle stages of trombiculid mites:
In temperate regions, adult females lay their eggs in ground vegetation in the Spring season once soils reach a specific temperature. After about a week, pre-larvae emerge from the eggs, but are as yet not fully formed. They require roughly one more week to develop their fourth leg, since they come into the world with only three. When they are fully developed larvae they are ready to feed on their host. These larvae are often referred to as "chiggers", which is a misnomer that comes from the chigoe flea. Interestingly, the larva stage of the mite life cycle is the only stage that is an ectoparasite. As nymphs and adults, the mites are not parasitic. The larvae typically await a passing host collectively, by agregating together in bunches in understory vegetation, including ground-based fallen vegetation and scrub and bush plants. These mite larvae are reddish in color, covered in hairs and very, very small. Here is a picture:
These mite larvae are not blood sucking arthropods. Rather, they feed on the enzymatically dissolved skin cells of their hosts. They accomplish this by first attaching to and piercing the skin and then injecting a cocktail of enzymes, which simultaneously anesthetizes the host and dissolves the tissue. It then sucks up this dissolved host tissue through the stylostome. The stylostome is actually a hollow tube that the larva has formed from the dead skins cells of the host. This structure along with the feeding mite larva are pictured here:
Since O. tsutsugamushi is transmitted transovarially between the adult female mite and her eggs, the larvae need not encounter an infected host to become infected. If the parent female was infected, the larvae will emerge infected and, upon reaching their first host, can immediately pass on the infection.
In some symptoms, the clinical presentation of scrub typhus is similar to that of epidemic and murine typhus, but it has a unique presenation as well. Headache, myaglia, sustained fever and a macular rash are similar symtpoms that scrub typhus shares with the other typhus forms. Its other symtpoms, however, diverge. To begin, scrub typhus typically presents with an initial eschar, which is an ulcer on the skin surface at the point of attachment for the mite larva ("chigger") that appears well after the larva has dropped off. Here is an example of a scrub typhus eschar:
Within days of the appearance of the eschar, other symptoms, such as headache, body ache, fever and sweating set in. Lymphadenopathy and splenomegaly may also begin to appear at this stage. Abdominal pain is also common in scrub typhus. Following, the maculopapular rash appears on the trunk and extends to the extremities, similar to the other typhus forms. Another unique feature is that scrub typhus can be hemorrhagic, with bleeding and coagulation pathologies present. Other complications include pneumonitis, encephalitis and myocarditis, all of which, if they do occur, present later in the disease course. The case-fatality associated with untreated scrub typhus varies dramatically across geography and pathogen strain, but can exhibit the highest associated mortality of all the typhus forms (range 1% to 60%). Older age is again associated with greater case-fatality.
The landscape epidemiology of scrub typhus is extraordinarily precise. Indeed, scrub typhus may be perhaps the most hyper-locally determined disease humans expereince. The overall global distribution forms the scrub typhus "triangle", wherein the northern boundary is Siberian Russia, the eastern boudary includes all of east and southeast Asia and extends well into the South Pacific islands, the southern boundary is northern Australia, and the western boundary extends beyond Afghanistan into central Asia. Here is a map of this global distribution:
But, as mentioned above, this is a disease defined by micro-geography, so any global geographic perspective of a scrub typhus "triangle" provides very little scope to help us epidemiologically delineate the transimission landscape of this disease.
First off, it is important to keep in mind that scrub typhus is a disease whose reservoir is the mite, but which can also act as a zoonotic disease by affecting rodents in a sylvan cycle of disease that commonly occurs in parallel with its natural reservoir. Human infection is accidental, and so, too, is infection in rodents. Nevertheless, the existence of a zoonotic sylvan cycle can amplify transmission in a given mite population.
Small communities of infected mite populations intersect with rodent populations to form small tsutsugamushi islands across the landscape. Indeed, some of these isolated infection zones can be only one meter square in size. These scrub typhus islands are detremined by the relevant reservoir mite populations, which in turn are shaped by precise fluctuations in soils, ground vegetation cover, temperature and humidity, and precipitation. Too moist, and the mites do not like the area. Too dry, and ground vegetation may be too sparse to support mite populations. Thus, extreme precision is required for transmission to humans. While these transmission islands define a high degree of landscape specificity for the occurrence of scrub typhus, they are not necessarily rare across the landscape depending on where in the global "triangle" they are located. Thus, this unique transmission potential has resulted in wide-spread epidemics under conditions of human encroachment on natural habitat characterized by overgrown vegetation. Particularly senstitive areas include forest and ecotones at marginally deforested locations and forrest clearings, as well as agricultural irrigation projects in arid or semi-arid regions. Workers in these settings and communities establishing new settlements tend to be at highest risk. Also, military conflict in these endemic landscapes, have been associated with very large outbreaks of scrub typhus in humans. This was a significant problem during World War II military campaigns in southeast Asia, and the South Pacific. In this setting, transmission to humans can be so extensive that as many as 50% of combatants are infected.
A more realistic global map of scrub typhus might look something like this map below, which was published in A Manual of Tropical Medicine in 1964. Needless to say, given that this is now 50 years old it may no longer be relevant, but it does highlight areas wherein the isolated islands of scrub typhus transmission might be commonly encountered:
Field surveys undertaking the remapping of scrub typhus across the traditional geographic "triangle" would be a very useful public health endeavor for the 21st century given the rapidly changing structures of populations across much of Asia and the concurrent escalation of human encroachment on natural habitat.