Tweet
This week we cover another virus in the Bunyaviridae family that causes hemorrhagic fever in humans: Crimean–Congo hemorrhagic fever virus. This is a zoonotic arbovirus vectored by ticks and demonstrates a wide geographic range from southern and eastern Europe, across the Middle East and western and central Asia, and down through sub-Saharan Africa. Human infection can be quite severe with high mortality.
The Pathogen. Crimean–Congo hemorrhagic fever (CCHF) is caused by Crimean–Congo hemorrhagic fever virus (CCHFV), which is a Nairovirus in the Bunyaviridae family. These viruses are approximately 80 to 120 nanometers in diameter. They are enveloped viruses with ambisense, single-stranded RNA genomes in three segments. The three genome segments are circular and are classified as large (L), medium (M), and small (S).
Macrophages, hepatocytes and endothelial cells are the target cells in the human host and, like hantaviruses, CCHFV invades host cells by endocytosis and replicates via the ER-Golgi intermediate compartment.
the hedgehogs (Erinaceinae subfamily):
and the European Hare (Lepus europaeus):
acting as the primary reservoirs throughout most of the range of this virus.
The Disease. Symptoms typically present with an abrupt onset and include fever, malaise, muscle pain, and headache early in the clinical course. Abdominal pain and nausea, with associated diarrhea and vomiting, respectively, can also present early on in the disease. Hepatomegaly is also a common clinical feature. As the disease progresses in subsequent days confusion and aggression commonly occur in concert with mood swings. Several bleeding symptoms often present with this disease. Petechiae commonly occur on the skin and in the mouth and throat. Intestinal bleeding can produce black stools or frank blood, and hematuria can present with or without visible discoloration. Bleeding from the nose and gums is quite common. By or before the end of the first week of illness, hepatitis is common and liver, renal, and/or pulmonary failure may ensue in those with a severe clinical course. By 10 to 14 days affected individuals begin to recover, but approximately 30% succumb to the infection.
The Epidemiology and the Landscape. CCHFV is transmitted to humans by the Hyalomma tick vector, as described above, from infected domestic livestock animals, or directly from person to person by way of contaminated blood or body fluid exposure. Vector-borne transmission is typically responsible for sporadic cases, whereas outbreaks typically follow from exposure to contaminated livestock during processing or consumption, or from exposure to contaminated body fluids during the care of infected patients in a clinical setting.
The map below produced by the World Health Organization (WHO) shows the global distribution of Hyalomma ticks (white and colors), viral- and seroprevalence (yellow), and incident cases (orange and red).
High concentrations of disease activity are apparent in parts of South, Central, and West Asia, and southeastern Europe. While the virus is present in much of Africa, significant numbers of human cases are only seen in South Africa, although this apparent anomaly could be due to under-reporting in several African countries.
The graphic below nicely depicts the key features of the landscape of CCHFV transmission and disease ecology.
The cycle above highlights different aspects of the complex landscape epidemiology of CCHF. The sylvan infection cycle is maintained by the tick vector and extraordinarily diverse small mammal populations in varied terrain, though many of these species prefer open field and fringe forest and scrub habitats. The same ticks that maintain the sylvan cycle also act as bridge vectors for transmission of CCHFV to livestock, or less commonly, directly to humans. Transmission to humans occurs most frequently following 1) exposure to their livestock or 2) exposure to each other in a health care setting following initial infection and presentation of index cases. Thus, animal husbandry and nosocomial transmission are the most significant conduits to human infection in the landscape even though direct transmission from ticks to humans remains a distinctly viable route of infection.
Control and Prevention. While most human infections in the outbreak setting are not attributable to tick bites, vector control is still a primary locus of prevention and control of human disease. It is generally comprised of two strategies.
The first vector control strategy is to take the usual precautions to prevent tick bites in humans and, thus, prevent sporadic cases. Use of long-sleeved shirts and long pants are very effective control measures as these reduce tick access to human skin. However, this approach may not be realistic for those that live or work outdoors in endemic areas during hotter months. As such, individuals who do spend time outside, and are at risk of exposure to ticks, should practice regular body tick checks.
Here is a nice video on the proper way to remove ticks from the skin:
The second vector control strategy is to prevent or minimize tick bites in domesticated livestock. This usually includes government regulated tick removal prior to the transport and processing of livestock animals. Acaricides are also frequently used and probably constitute the largest agricultural prevention strategy in many areas. However, both the above livestock vector control methods require significant resources and good livestock production infrastructure, neither of which are often available for poor subsistence farmers and herders in many areas where CCHFV is endemic.
Another important non-vector control strategy employs good barrier protection and patient isolation to prevent nosocomial spread from infected patients to health care personnel and/or other non-infected patients in a hospital or health care setting. This is a critical component to CCHFV control and prevention as outbreaks often generate many secondary cases by human to human transmission during the care of infected individuals.
This week we cover another virus in the Bunyaviridae family that causes hemorrhagic fever in humans: Crimean–Congo hemorrhagic fever virus. This is a zoonotic arbovirus vectored by ticks and demonstrates a wide geographic range from southern and eastern Europe, across the Middle East and western and central Asia, and down through sub-Saharan Africa. Human infection can be quite severe with high mortality.
The Pathogen. Crimean–Congo hemorrhagic fever (CCHF) is caused by Crimean–Congo hemorrhagic fever virus (CCHFV), which is a Nairovirus in the Bunyaviridae family. These viruses are approximately 80 to 120 nanometers in diameter. They are enveloped viruses with ambisense, single-stranded RNA genomes in three segments. The three genome segments are circular and are classified as large (L), medium (M), and small (S).
Crimean–Congo hemorrhagic fever virus (Published in Antiviral Research, Volume 64, Issue 3, December 2004, Pages 145-160)
Macrophages, hepatocytes and endothelial cells are the target cells in the human host and, like hantaviruses, CCHFV invades host cells by endocytosis and replicates via the ER-Golgi intermediate compartment.
Bunyaviridae infection cycle (Published in: Antiviral Research, Volume 64, Issue 3, December 2004, Pages 145-160)
The Vector. CCHFV is vectored by ticks between reservoir hosts, which maintains the natural sylvan infection cycle. However this vector is also very important in introducing the infection to domestic livestock. While tick-borne transmission is a relevant pathway to infection in humans, this mode of transmission generally accounts for sporadic human cases only. On the other hand, outbreaks in humans typically result from contact with infected livestock, which in turn are infected by ticks.
The most important tick vectors for CCHFV transmission are those of the genus Hyalomma.
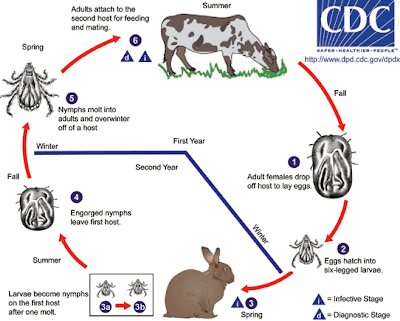
These are hard-bodied ticks in the family Ixodidae. Unlike other hard-bodied ticks, the Hyalomma leave a moderately serious bite wound after detaching, which can often necrotize the surrounding tissue. These ticks are widely distributed throughout Europe, Africa, and Asia. The graphic below developed by the Centers for Disease Control and Prevention (CDC) nicely depicts the two year life cycle of the Hyalomma ticks.
The Reservoir. Small mammals comprise the natural reservoir hosts of CCHFV, with rodents in the genus Mastomys:The most important tick vectors for CCHFV transmission are those of the genus Hyalomma.
Hyalomma marginatum
These are hard-bodied ticks in the family Ixodidae. Unlike other hard-bodied ticks, the Hyalomma leave a moderately serious bite wound after detaching, which can often necrotize the surrounding tissue. These ticks are widely distributed throughout Europe, Africa, and Asia. The graphic below developed by the Centers for Disease Control and Prevention (CDC) nicely depicts the two year life cycle of the Hyalomma ticks.
the hedgehogs (Erinaceinae subfamily):
and the European Hare (Lepus europaeus):
acting as the primary reservoirs throughout most of the range of this virus.
The Disease. Symptoms typically present with an abrupt onset and include fever, malaise, muscle pain, and headache early in the clinical course. Abdominal pain and nausea, with associated diarrhea and vomiting, respectively, can also present early on in the disease. Hepatomegaly is also a common clinical feature. As the disease progresses in subsequent days confusion and aggression commonly occur in concert with mood swings. Several bleeding symptoms often present with this disease. Petechiae commonly occur on the skin and in the mouth and throat. Intestinal bleeding can produce black stools or frank blood, and hematuria can present with or without visible discoloration. Bleeding from the nose and gums is quite common. By or before the end of the first week of illness, hepatitis is common and liver, renal, and/or pulmonary failure may ensue in those with a severe clinical course. By 10 to 14 days affected individuals begin to recover, but approximately 30% succumb to the infection.
Patient with severe petechial rashes (ecchymoses)
The Epidemiology and the Landscape. CCHFV is transmitted to humans by the Hyalomma tick vector, as described above, from infected domestic livestock animals, or directly from person to person by way of contaminated blood or body fluid exposure. Vector-borne transmission is typically responsible for sporadic cases, whereas outbreaks typically follow from exposure to contaminated livestock during processing or consumption, or from exposure to contaminated body fluids during the care of infected patients in a clinical setting.
The map below produced by the World Health Organization (WHO) shows the global distribution of Hyalomma ticks (white and colors), viral- and seroprevalence (yellow), and incident cases (orange and red).
High concentrations of disease activity are apparent in parts of South, Central, and West Asia, and southeastern Europe. While the virus is present in much of Africa, significant numbers of human cases are only seen in South Africa, although this apparent anomaly could be due to under-reporting in several African countries.
The graphic below nicely depicts the key features of the landscape of CCHFV transmission and disease ecology.
Sylvan and Human Infection Cycles (Published in Perspectives in Medical Virology, Elsevier, 2006, Volume 16, Pages 299-324)
The cycle above highlights different aspects of the complex landscape epidemiology of CCHF. The sylvan infection cycle is maintained by the tick vector and extraordinarily diverse small mammal populations in varied terrain, though many of these species prefer open field and fringe forest and scrub habitats. The same ticks that maintain the sylvan cycle also act as bridge vectors for transmission of CCHFV to livestock, or less commonly, directly to humans. Transmission to humans occurs most frequently following 1) exposure to their livestock or 2) exposure to each other in a health care setting following initial infection and presentation of index cases. Thus, animal husbandry and nosocomial transmission are the most significant conduits to human infection in the landscape even though direct transmission from ticks to humans remains a distinctly viable route of infection.
Control and Prevention. While most human infections in the outbreak setting are not attributable to tick bites, vector control is still a primary locus of prevention and control of human disease. It is generally comprised of two strategies.
The first vector control strategy is to take the usual precautions to prevent tick bites in humans and, thus, prevent sporadic cases. Use of long-sleeved shirts and long pants are very effective control measures as these reduce tick access to human skin. However, this approach may not be realistic for those that live or work outdoors in endemic areas during hotter months. As such, individuals who do spend time outside, and are at risk of exposure to ticks, should practice regular body tick checks.
Here is a nice video on the proper way to remove ticks from the skin:
The second vector control strategy is to prevent or minimize tick bites in domesticated livestock. This usually includes government regulated tick removal prior to the transport and processing of livestock animals. Acaricides are also frequently used and probably constitute the largest agricultural prevention strategy in many areas. However, both the above livestock vector control methods require significant resources and good livestock production infrastructure, neither of which are often available for poor subsistence farmers and herders in many areas where CCHFV is endemic.
Another important non-vector control strategy employs good barrier protection and patient isolation to prevent nosocomial spread from infected patients to health care personnel and/or other non-infected patients in a hospital or health care setting. This is a critical component to CCHFV control and prevention as outbreaks often generate many secondary cases by human to human transmission during the care of infected individuals.