Tweet
This time at Infection Landscapes we cover the "madness" disease. Rabies is a zoonotic disease that causes encephalitis and, if no vaccine is administered, almost always results in death. While human rabies infection is not common in the developed world, it is a significant cause of death in the developing world where the vast majority of infections are acquired from dogs. The name "rabies" comes from the Latin word for madness. This is an ancient disease having been documented for almost four thousand years. It has always been associated with rage and violence in the afflicted.
The Pathogen: Rabies is caused by the rabies virus, which is the type species for the Lyssavirus genus in the Rhabdoviridae family. Rabies virus is an enveloped, single-stranded negative-sense RNA virus with a helical capsid. The virus is approximately 75 nm in diameter and 180 nm long.
The primary target host cells are neurons. Each stage of the rabies virus infection cycle in the host cell is illustrated in the graphic below (published in Lancet Infectious Diseases Volume 2, Issue 6, June 2002, Pages 327–343).
The virus first gains the peripheral nervous system where it passes along axons in the periphery until it eventually gains the central nervous system, which is the primary site of pathogenesis. The graphic below depicts the pathogenic cycle in animal rabies (published in Lancet Infectious Diseases Volume 2, Issue 6, June 2002, Pages 327–343):
The Disease. Rabies is an extremely serious disease, which almost always results in death if infected individuals do not receive post-exposure prophylaxis before symtpoms begin to appear. The incubation period is highly varied and can range from several days to several years, however for most human infections symptoms typically appear between 2 and 12 weeks following infection. Initial symptoms are often vague and may be comprised of only fever and general flu-like illness. However, this progresses rapidly to include neurologic sequelae.
There are two general forms of clinical rabies in both animals and humans: furious rabies, and paralytic rabies. Furious rabies occurs in approximately 70% of human rabies cases and presents first with a prodrome typified by feelings of great anxiety. Other symptoms during this phase are non-specific and may include headache, myalgia, and fever. Following the prodrome, the neurologic phase progresses with intermittent agitation and aggressive behavior. Hydrophobia is a common symptom due to spasms of muscle structures in the neck. Increased saliva is also a prominent feature of the neurologic phase. Coma then develops in a short time, typically within a few days from the onset of this phase. Other autonomic abnormalities are common and can include pulmonary edema, blood pressure variability, and cardiac arrhythmia. Interestingly, there can also be differences in presentation based on whether the human infection was acquired from a dog or a bat, the latter being associated with seizures and hallucinations. Paralytic rabies occurs in approximately 30% of humans rabies cases, and is much more difficult to identify and diagnose. This form of disease is very similar in clinical appearance to Guillain-Barre syndrome with respect to encephalopathy, paralysis, and electrophysiological findings.
Post exposure prophylaxis (PEP) is critical to administer to any individual suspected of having been inoculated with the rabies virus. There are two primary components to PEP. First, the bite wound, or suspected point of entry, must be thoroughly cleaned using soap and water as soon as possible after exposure. This can help to reduce the number of virus particles that can gain entry into cells. Second, vaccination must be provided as soon as possible following exposure. Rabies is one of the very few infections for which effective immunization can be administered after the exposure. This is due to the extended period of time required for the virus particles to reach the CNS and initiate pathogenesis. As such, PEP can be administered during the incubation period to effectively block pathogenesis. However, while the incubation period is typically a few weeks, it can be as short as a few days for some individuals. As such, it is critical to begin PEP as soon as possible following the exposure. In addition, the number of virions introduced to the infected individual as well as the site of introduction are important determinants of the duration of the incubation period. For example, individuals with multiple or severe bite wounds will typically have a larger inoculum, and those with bites on the hands, face, neck, or head will have a shorter distance for virions to travel before reaching the CNS. In either scenario, the incubation period can be reduced substantially. Children are also at greater risk because they typically experience bites on the face and head more frequently than adults.
A course of tissue culture vaccine should be administered, however it takes 1 to 2 weeks to mount an adequate humoral immune response. As such, the necessary antibodies may not be available in time for individuals with short incubation periods. Therefore, in addition to the tissue culture vaccine, we rely first on passive immunity by administering antirabies immunoglobulin (RIG) in and around all bite wounds. The passive antibodies must be injected at the wound sites, not intramuscularly at some site distal to the wounds.
The document below states the specific recommendations of the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC):
Rabies Post-Exposure Prophylaxis Schedule (PDF)
The problem with PEP is that it is expensive and often not available at all, or in part, in those areas of the world with high endemicity of rabies in dogs, particularly South and Southeast Asia and Africa. Thus, even if a rabies exposure is well documented and identified prior to the onset of symptoms, often supplies are inadequate to administer complete and effective PEP.
As mentioned above, in the developed world and in the United States in particular, almost all rabies infections are due to contact with wild animals. Therefore, the best prevention strategy for rabies in these areas is to avoid contact with wild animals. This is a good general practice for many reasons, but it also serves to remove the potential for rabies transmission between infected animals and humans. And we should remember that contact with wildlife is much more likely in an urban landscape than a sylvan landscape for most people. For example, there is a large raccoon population in Manhattan in the heart of New York City and many of these animals have tested positive for rabies. One should be especially wary of any animal that is exhibiting unusual, erratic, or aggressive behavior. If any contact with such an animal does occur, seek medical consultation.
This time at Infection Landscapes we cover the "madness" disease. Rabies is a zoonotic disease that causes encephalitis and, if no vaccine is administered, almost always results in death. While human rabies infection is not common in the developed world, it is a significant cause of death in the developing world where the vast majority of infections are acquired from dogs. The name "rabies" comes from the Latin word for madness. This is an ancient disease having been documented for almost four thousand years. It has always been associated with rage and violence in the afflicted.
The Pathogen: Rabies is caused by the rabies virus, which is the type species for the Lyssavirus genus in the Rhabdoviridae family. Rabies virus is an enveloped, single-stranded negative-sense RNA virus with a helical capsid. The virus is approximately 75 nm in diameter and 180 nm long.
The primary target host cells are neurons. Each stage of the rabies virus infection cycle in the host cell is illustrated in the graphic below (published in Lancet Infectious Diseases Volume 2, Issue 6, June 2002, Pages 327–343).
The virus first gains the peripheral nervous system where it passes along axons in the periphery until it eventually gains the central nervous system, which is the primary site of pathogenesis. The graphic below depicts the pathogenic cycle in animal rabies (published in Lancet Infectious Diseases Volume 2, Issue 6, June 2002, Pages 327–343):
The Disease. Rabies is an extremely serious disease, which almost always results in death if infected individuals do not receive post-exposure prophylaxis before symtpoms begin to appear. The incubation period is highly varied and can range from several days to several years, however for most human infections symptoms typically appear between 2 and 12 weeks following infection. Initial symptoms are often vague and may be comprised of only fever and general flu-like illness. However, this progresses rapidly to include neurologic sequelae.
There are two general forms of clinical rabies in both animals and humans: furious rabies, and paralytic rabies. Furious rabies occurs in approximately 70% of human rabies cases and presents first with a prodrome typified by feelings of great anxiety. Other symptoms during this phase are non-specific and may include headache, myalgia, and fever. Following the prodrome, the neurologic phase progresses with intermittent agitation and aggressive behavior. Hydrophobia is a common symptom due to spasms of muscle structures in the neck. Increased saliva is also a prominent feature of the neurologic phase. Coma then develops in a short time, typically within a few days from the onset of this phase. Other autonomic abnormalities are common and can include pulmonary edema, blood pressure variability, and cardiac arrhythmia. Interestingly, there can also be differences in presentation based on whether the human infection was acquired from a dog or a bat, the latter being associated with seizures and hallucinations. Paralytic rabies occurs in approximately 30% of humans rabies cases, and is much more difficult to identify and diagnose. This form of disease is very similar in clinical appearance to Guillain-Barre syndrome with respect to encephalopathy, paralysis, and electrophysiological findings.
Post exposure prophylaxis (PEP) is critical to administer to any individual suspected of having been inoculated with the rabies virus. There are two primary components to PEP. First, the bite wound, or suspected point of entry, must be thoroughly cleaned using soap and water as soon as possible after exposure. This can help to reduce the number of virus particles that can gain entry into cells. Second, vaccination must be provided as soon as possible following exposure. Rabies is one of the very few infections for which effective immunization can be administered after the exposure. This is due to the extended period of time required for the virus particles to reach the CNS and initiate pathogenesis. As such, PEP can be administered during the incubation period to effectively block pathogenesis. However, while the incubation period is typically a few weeks, it can be as short as a few days for some individuals. As such, it is critical to begin PEP as soon as possible following the exposure. In addition, the number of virions introduced to the infected individual as well as the site of introduction are important determinants of the duration of the incubation period. For example, individuals with multiple or severe bite wounds will typically have a larger inoculum, and those with bites on the hands, face, neck, or head will have a shorter distance for virions to travel before reaching the CNS. In either scenario, the incubation period can be reduced substantially. Children are also at greater risk because they typically experience bites on the face and head more frequently than adults.
A course of tissue culture vaccine should be administered, however it takes 1 to 2 weeks to mount an adequate humoral immune response. As such, the necessary antibodies may not be available in time for individuals with short incubation periods. Therefore, in addition to the tissue culture vaccine, we rely first on passive immunity by administering antirabies immunoglobulin (RIG) in and around all bite wounds. The passive antibodies must be injected at the wound sites, not intramuscularly at some site distal to the wounds.
The document below states the specific recommendations of the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC):
Rabies Post-Exposure Prophylaxis Schedule (PDF)
The problem with PEP is that it is expensive and often not available at all, or in part, in those areas of the world with high endemicity of rabies in dogs, particularly South and Southeast Asia and Africa. Thus, even if a rabies exposure is well documented and identified prior to the onset of symptoms, often supplies are inadequate to administer complete and effective PEP.
Epidemiology and the Landscape. Rabies virus is shed in the saliva of symptomatic hosts and, as such, contact transmission via biting is the primary route of infection. Rabies virus only infects mammals and, while all mammals are susceptible to infection, certain mammal species are particularly important as reservoir hosts and vectors for human infection. In particular, dogs, cats, domestic livestock, and bats are important sources of rabies infection to humans. Dogs are by the most important source of human infection, accounting for the vast majority of human infections worldwide (however, almost all human rabies infections in the United States are due to contact with wild animals, particularly raccoons, skunks, and bats). Areas with large populations of free roaming stray dogs have the largest occurrence of rabies in humans. The single most important feature defining the landscape of human rabies infection is the spatial range of stray dogs. This feature is definitive because the range of stray dogs always intersects or is in union with the human social landscape in either its residential or occupational components, or both. Moreover, the spatial range of stray dogs often is in contact with sylvan or peri-sylvan landscapes at its boundaries, thus maintaining both the primary domestic rabies reservoir, through contact between dogs and other sylvan mammals, and the human transmission cycle, through regular contact between dogs and humans. The map below shows the important mammal reservoirs and vectors for rabies virus (published in Lancet Infectious Diseases Volume 2, Issue 6, June 2002, Pages 327–343).
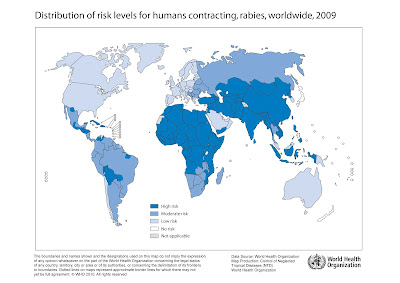
Rabies exists in almost every country and landmass on earth with the exception of Antarctica and some remote islands. The regions of South Asia and sub-Saharan Africa experience the vast majority of human rabies each year, with India alone accounting for approximately 20,000 of the total 55,000 annual rabies deaths. The map below produced by the World Health Organization (WHO) shows the global distribution of rabies infection risk for humans:
The transmission of rabies by bite from sylvan hosts to domestic livestock is another important route of transmission that can result in secondary transmission to human hosts. In addition, airborne transmission is a possible, but rare, route of infection that can occur if the saliva of an infected host becomes aerosolized and is subsequently inhaled by a susceptible host. Such cases have been documented among individuals exploring caves that harbor bats, or among people with bats in the home who were not bitten. However, airborne transmission of rabies is exceptionally rare, at least among documented cases, so it is difficult to know exactly whether or not transmission to humans with no history of a bat bite acquired an aerosolized infection or acquired the infection through the typical biting route, but from a bite that was imperceptible as a bite. Indeed, it is not uncommon to experience a bite from some bat species and be unaware of the incident, especially if the bite was acquired during sleep and is not in a conspicuous position on the body.
Control and Prevention. Control of animal vectors is the primary strategy for the prevention of rabies in humans. This strategy typically targets dogs, as these are the most important animal vector for transmission to humans, and involves two intervention components. The first is the management of stray dog populations to reduce numbers, which may entail culling. The management of stray dog populations is critical because 1) it reduces the reservoir population and the potential effective contacts between stray dogs and humans, and 2) smaller, managed populations of dogs can be more easily administered the second intervention component. The second component consists of canine vaccination. Rabies can be controlled in the canine population if 70% of dogs are vaccinated using inactivated virus vaccine. Immunity lasts for approximately 3 years in the animals.
As mentioned above, in the developed world and in the United States in particular, almost all rabies infections are due to contact with wild animals. Therefore, the best prevention strategy for rabies in these areas is to avoid contact with wild animals. This is a good general practice for many reasons, but it also serves to remove the potential for rabies transmission between infected animals and humans. And we should remember that contact with wildlife is much more likely in an urban landscape than a sylvan landscape for most people. For example, there is a large raccoon population in Manhattan in the heart of New York City and many of these animals have tested positive for rabies. One should be especially wary of any animal that is exhibiting unusual, erratic, or aggressive behavior. If any contact with such an animal does occur, seek medical consultation.
Photo by umbrooklynborn.blogspot.com