Tweet
This time on Infection Landscapes we will cover another zoonotic infection that has afflicted humans and their domestic livestock probably for thousands of years: anthrax. This discussion will focus only on zoonotic infections in humans, and to a lesser extent enzootic and epizootic transmission among animals. The use of anthrax as a biological weapon will not be covered here as this does not fall within the purview of Infection Landscapes.
The Pathogen: Anthrax is caused by the gram-positive bacterium, Bacillus anthracis. As indicated by the genus name, this is a bacillary, or rod-shaped, bacterium:
The organism is spore-forming and metabolically aerobic when active. The spores are extremely robust to environmental insult and, as such, can last for many years in the environment until favorable conditions emerge. B. anthracis initially targets macrophages in the host. Pathogenicity and virulence are driven specifically by the presence the bacterium's capsule and its production of endotoxins. The capsule prevents phagocytosis by macrophages and other phagocytic cells, and thus is able to evade an important arm of innate immunity in the host. The endotoxins are directly responsible for pathogenesis: edema factor mediates an increase in intracellular cAMP, which leads to severe edema in tissues, while lethal factor leads to tissue necrosis. Subsequently, the bacilli can be disseminated by way of the blood and lymph circulation.
The Disease. Anthrax typically falls into one of three distinct categories of clinical presentation: cutaneous, pulmonary, and gastrointestinal.
Cutaneous anthrax is the most common presentation in humans. Cutaneous anthrax typically presents with an abscess on the skin, which quickly progresses to a black eschar of necrotized tissue:
This kind of presentation generally follows transmission of the spores via open breaks or wounds in the skin following the handling or processing of infected animals. If treated effectively, cutaneous anthrax typically remains localized without generating a potentially life threatening toxemia. However, if cutaneous anthrax is not treated then the disease is subsequently associated with approximately 20% case fatality.
Pulmonary anthrax is a much more severe form of disease. Early presentation mimics an influenza-like illness, with fever, malaise, and cough. However, this can progress to fulminant disease and ultimate respiratory collapse, which is associated with a case fatality greater than 95%. If treated early, i.e. before fulminant disease ensues, this case fatality can be reduced by half. Pulmonary anthrax follows the inhalation of anthrax spores and is therefore known as "inhalation anthrax". However, the infective dose of pulmonary anthrax is much higher than for cutaneous anthrax (~10,000-20,000 spores).
Gastrointestinal anthrax is the least common disease form, but is also quite severe. Severe inflammation and lesions can present throughout the entire alimentary canal. Severe diarrhea and vomiting, both with or without blood, and anorexia are common early and persistent symptoms. The greatest threat from gastrointestinal anthrax results from the bacilli gaining the circulation from the gut tract, which can subsequently lead to a life threatening toxemia. Gastrointestinal anthrax typically follows the consumption of meat infected with B. anthracis. The case fatality associated with gastrointestinal anthrax can range from approximately 25% if treatment is initiated early to approximately 60% if treatment is significantly delayed.
The Epidemiology and the Landscape. There are three primary modes of transmission for B. anthracis infection in humans. Direct contact between breaks or wounds in the skin and spores in the environment or via infected animals is probably the most common route for human infection. Infection via this route is associated with cutaneous anthrax. Airborne transmission is possible when the spores are inhaled and subsequently initiate infection in the lungs, which has been associated with processing in certain kinds of animal industry. Infection via this route leads to pulmonary anthrax. Finally, food-borne transmission results from the consumption of contaminated meat. This mode of transmission is probably the least common in most endemic areas, however community outbreaks of gastrointestinal anthrax can be quite large particularly if infection is widespread among a herd of cattle slaughtered for food.
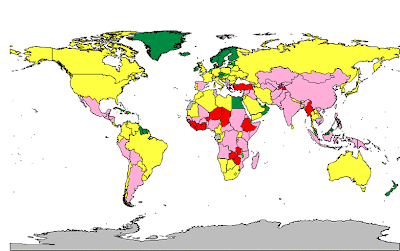
Global surveillance on anthrax incidence is quite poor, particularly in endemic areas. The map below, produced by the World Health Organization Collaborating Center for Remote Sensing and Geographic Information Systems for Public Health, depicts the general areas of endemicity by country across the world:
While the absolute numbers of incident cases are not likely to be high on an annual basis, large geographic areas of endemicity are apparent across much of the world including Central America, the Andean region of South America, sub-Saharan Africa, and Central, South, East, and Southeast Asia.
The landscape of anthrax is one delineated primarily by the spaces of interaction between humans and domestic livestock. More importantly, the specific kinds of animal processing in different kinds of industry define the types of interaction that can take place, and which may lead to transmission between infected animals, or their infectious products, and susceptible human hosts. The graphic below, prepared by the World Health Organization, demonstrates the primary routes of infection transmission (note: the route of vectored transmission by biting fly depicted by the dashed line is largely theoretical):
Where anthrax remains endemic, it typically infects the grass-eating bovids, domestic or sylvan, which encounter spores in the environment as they graze. Individuals working in industries that process livestock animals, such as cattle, goats, and sheep, in these endemic areas are at greatest risk of infection. More specifically, the direct handling of animal products in this setting is a common source of exposure. For example, the processing of animal hides for the production of clothing or other products that use these materials, such as drums, etc, can lead to contact transmission and subsequent cutaneous anthrax disease. Individuals involved with the processing of wool from sheep have historically experienced the highest risk of any occupation due to the significant potential for inhalation of the airborne spores in dust particles during the sheering of infected sheep as well as during the subsequent textile production. The latter route of transmission is quite uncommon today because most areas of the world where sheep husbandry is an important industry have eliminated anthrax in their animal populations. The two examples above illustrate how the landscape of infection can be comprised of intersecting physical and social spaces. In other words, risk of infection is not limited to those with direct contact with infected animals, but rather can extend to those who work with the products of those animals in occupational spaces potentially far removed from the original source of the pathogen.
Additionally, an outbreak of anthrax has been reported among injection drug users. Transmission in this scenario resulted from heroin contaminated with bone meal from infected animals. The bone meal is used to cut the drug prior to distribution.
Control and Prevention. Animal vaccines for livestock have been the cornerstone of anthrax prevention for more than one hundred years. Effective vaccination campaigns have reduced the burden of anthrax in livestock, and secondarily in people, in many parts of the world. The work of Louis Pasteur in the late 19th century, and then later Max Sterne in 1935, contributed to effective vaccination against anthrax in livestock animals. Louis Pasteur's anthrax vaccine was the first vaccine developed against a bacterium, and only the second vaccine produced up to that point in time (the first vaccine was developed by Edward Jenner against smallpox). Indeed, Louis Pasteur coined the term "vaccine". While Pasteur's vaccine was quite effective against infection with B. anthracis, it was also quite difficult to effect minimal virulence and maximal immunogenicity during the processing of this live-attenuated vaccine. Sterne was able to isolate a particular strain of B. anthracis that was much more amenable to efficient vaccine production and remains the current vaccine of choice for the protection of livestock in endemic areas of the world.
Human vaccines are also available in the United States, Europe, and China, but these are primarily used in military personnel among whom exposure to B. anthracis as a biological weapon may be anticipated. However, as described above, this form of anthrax transmission is beyond the scope of Infection Landscapes and so will not be discussed here.
Finally, the carcasses of animals killed by anthrax can be a significant public health threat if left unattended in the landscape. Specific measures to contain B. anthracis and prevent any further spread of the bacteria are critical interventions.
These carcasses must be removed to prevent contact with sylvan scavengers as well as to prevent contact with, and further dispersal within, the grazing lands of other domestic livestock. Burning or burying of infected dead animals are common methods that have been used for centuries. However, these are by no means fail proof. In particular, complete burning of an infected animal (which would be required to eliminate all pathogenic bacilli) can take days. Such a fuel-intensive process may not be feasible in many, or perhaps most, areas of the world where anthrax is endemic. Burying is also a difficult solution because this must be done at quite a substantial depth to prevent the re-emergence of spores due to subsequent weathering and/or other erosional forces. It is important to keep in mind that if the spores are not destroyed they can remain viable in the environment for many years.
This time on Infection Landscapes we will cover another zoonotic infection that has afflicted humans and their domestic livestock probably for thousands of years: anthrax. This discussion will focus only on zoonotic infections in humans, and to a lesser extent enzootic and epizootic transmission among animals. The use of anthrax as a biological weapon will not be covered here as this does not fall within the purview of Infection Landscapes.
The Pathogen: Anthrax is caused by the gram-positive bacterium, Bacillus anthracis. As indicated by the genus name, this is a bacillary, or rod-shaped, bacterium:
Bacillus anthracis rods
The organism is spore-forming and metabolically aerobic when active. The spores are extremely robust to environmental insult and, as such, can last for many years in the environment until favorable conditions emerge. B. anthracis initially targets macrophages in the host. Pathogenicity and virulence are driven specifically by the presence the bacterium's capsule and its production of endotoxins. The capsule prevents phagocytosis by macrophages and other phagocytic cells, and thus is able to evade an important arm of innate immunity in the host. The endotoxins are directly responsible for pathogenesis: edema factor mediates an increase in intracellular cAMP, which leads to severe edema in tissues, while lethal factor leads to tissue necrosis. Subsequently, the bacilli can be disseminated by way of the blood and lymph circulation.
The Disease. Anthrax typically falls into one of three distinct categories of clinical presentation: cutaneous, pulmonary, and gastrointestinal.
Cutaneous anthrax is the most common presentation in humans. Cutaneous anthrax typically presents with an abscess on the skin, which quickly progresses to a black eschar of necrotized tissue:
This kind of presentation generally follows transmission of the spores via open breaks or wounds in the skin following the handling or processing of infected animals. If treated effectively, cutaneous anthrax typically remains localized without generating a potentially life threatening toxemia. However, if cutaneous anthrax is not treated then the disease is subsequently associated with approximately 20% case fatality.
Pulmonary anthrax is a much more severe form of disease. Early presentation mimics an influenza-like illness, with fever, malaise, and cough. However, this can progress to fulminant disease and ultimate respiratory collapse, which is associated with a case fatality greater than 95%. If treated early, i.e. before fulminant disease ensues, this case fatality can be reduced by half. Pulmonary anthrax follows the inhalation of anthrax spores and is therefore known as "inhalation anthrax". However, the infective dose of pulmonary anthrax is much higher than for cutaneous anthrax (~10,000-20,000 spores).
Gastrointestinal anthrax is the least common disease form, but is also quite severe. Severe inflammation and lesions can present throughout the entire alimentary canal. Severe diarrhea and vomiting, both with or without blood, and anorexia are common early and persistent symptoms. The greatest threat from gastrointestinal anthrax results from the bacilli gaining the circulation from the gut tract, which can subsequently lead to a life threatening toxemia. Gastrointestinal anthrax typically follows the consumption of meat infected with B. anthracis. The case fatality associated with gastrointestinal anthrax can range from approximately 25% if treatment is initiated early to approximately 60% if treatment is significantly delayed.
The Epidemiology and the Landscape. There are three primary modes of transmission for B. anthracis infection in humans. Direct contact between breaks or wounds in the skin and spores in the environment or via infected animals is probably the most common route for human infection. Infection via this route is associated with cutaneous anthrax. Airborne transmission is possible when the spores are inhaled and subsequently initiate infection in the lungs, which has been associated with processing in certain kinds of animal industry. Infection via this route leads to pulmonary anthrax. Finally, food-borne transmission results from the consumption of contaminated meat. This mode of transmission is probably the least common in most endemic areas, however community outbreaks of gastrointestinal anthrax can be quite large particularly if infection is widespread among a herd of cattle slaughtered for food.
Global surveillance on anthrax incidence is quite poor, particularly in endemic areas. The map below, produced by the World Health Organization Collaborating Center for Remote Sensing and Geographic Information Systems for Public Health, depicts the general areas of endemicity by country across the world:
The landscape of anthrax is one delineated primarily by the spaces of interaction between humans and domestic livestock. More importantly, the specific kinds of animal processing in different kinds of industry define the types of interaction that can take place, and which may lead to transmission between infected animals, or their infectious products, and susceptible human hosts. The graphic below, prepared by the World Health Organization, demonstrates the primary routes of infection transmission (note: the route of vectored transmission by biting fly depicted by the dashed line is largely theoretical):
Where anthrax remains endemic, it typically infects the grass-eating bovids, domestic or sylvan, which encounter spores in the environment as they graze. Individuals working in industries that process livestock animals, such as cattle, goats, and sheep, in these endemic areas are at greatest risk of infection. More specifically, the direct handling of animal products in this setting is a common source of exposure. For example, the processing of animal hides for the production of clothing or other products that use these materials, such as drums, etc, can lead to contact transmission and subsequent cutaneous anthrax disease. Individuals involved with the processing of wool from sheep have historically experienced the highest risk of any occupation due to the significant potential for inhalation of the airborne spores in dust particles during the sheering of infected sheep as well as during the subsequent textile production. The latter route of transmission is quite uncommon today because most areas of the world where sheep husbandry is an important industry have eliminated anthrax in their animal populations. The two examples above illustrate how the landscape of infection can be comprised of intersecting physical and social spaces. In other words, risk of infection is not limited to those with direct contact with infected animals, but rather can extend to those who work with the products of those animals in occupational spaces potentially far removed from the original source of the pathogen.
Additionally, an outbreak of anthrax has been reported among injection drug users. Transmission in this scenario resulted from heroin contaminated with bone meal from infected animals. The bone meal is used to cut the drug prior to distribution.
Control and Prevention. Animal vaccines for livestock have been the cornerstone of anthrax prevention for more than one hundred years. Effective vaccination campaigns have reduced the burden of anthrax in livestock, and secondarily in people, in many parts of the world. The work of Louis Pasteur in the late 19th century, and then later Max Sterne in 1935, contributed to effective vaccination against anthrax in livestock animals. Louis Pasteur's anthrax vaccine was the first vaccine developed against a bacterium, and only the second vaccine produced up to that point in time (the first vaccine was developed by Edward Jenner against smallpox). Indeed, Louis Pasteur coined the term "vaccine". While Pasteur's vaccine was quite effective against infection with B. anthracis, it was also quite difficult to effect minimal virulence and maximal immunogenicity during the processing of this live-attenuated vaccine. Sterne was able to isolate a particular strain of B. anthracis that was much more amenable to efficient vaccine production and remains the current vaccine of choice for the protection of livestock in endemic areas of the world.
Human vaccines are also available in the United States, Europe, and China, but these are primarily used in military personnel among whom exposure to B. anthracis as a biological weapon may be anticipated. However, as described above, this form of anthrax transmission is beyond the scope of Infection Landscapes and so will not be discussed here.
Finally, the carcasses of animals killed by anthrax can be a significant public health threat if left unattended in the landscape. Specific measures to contain B. anthracis and prevent any further spread of the bacteria are critical interventions.
These carcasses must be removed to prevent contact with sylvan scavengers as well as to prevent contact with, and further dispersal within, the grazing lands of other domestic livestock. Burning or burying of infected dead animals are common methods that have been used for centuries. However, these are by no means fail proof. In particular, complete burning of an infected animal (which would be required to eliminate all pathogenic bacilli) can take days. Such a fuel-intensive process may not be feasible in many, or perhaps most, areas of the world where anthrax is endemic. Burying is also a difficult solution because this must be done at quite a substantial depth to prevent the re-emergence of spores due to subsequent weathering and/or other erosional forces. It is important to keep in mind that if the spores are not destroyed they can remain viable in the environment for many years.